Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL3: Carbon (13c) Nmr Spectroscopy
Section: Chapter Questions
Problem 5E
Related questions
Question
please explain with reason
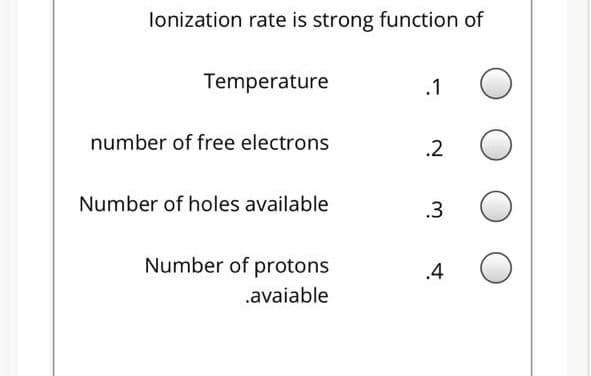
Transcribed Image Text:lonization rate is strong function of
Temperature
.1
number of free electrons
.2
Number of holes available
.3
Number of protons
.4
.avaiable
Expert Solution
Step 1
The Ionization rate (J) is given by the relation as-
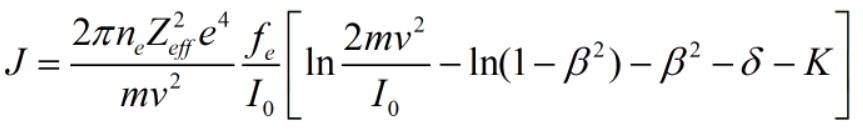
fe: effective fraction.
v: velocity of particle.
ne: number of electrons.
I0: ionization potential.
β: particle velocity.
Zeff: effective charge.
δ: coreection term.
m: mass of the particle.
Step 2
from the above relation it is clear that ionization rate is directly proportional to the number of electrons involved in the particular reaction.
Therefore, Ionization rate is a strong function of number of free electrons only.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole