Many metabolites are maintained at steady-state concentrations that are far from equilibrium. A comparison of Kéq and Q, the mass-action ratio, can determine whether a metabolic reaction is far from equilibrium. The equation for this equilibrium is, fructose 6-phosphate + ATP = fructose 1,6-bisphosphate + ADP Calculate Ke, for this reaction at T = 25.0 °C. AG' = -14.2 kJ/mol Calculate the mass-action ratio, Q, from the approximate physiological concentrations for rat heart tissue shown in the table. Metabolite Concentration (µM) Q = fructose 6-phosphate 84.0 fructose 1,6-bisphosphate 25.0 АТР 12,100 ADP 1,280 Select the true statements about the PFK-1 reaction. The PFK-1 reaction in heart tissue does not reach equilibrium. In heart tissue, the PFK-1 reaction will be driven toward product formation. In heart tissue, the PFK-1 products are more abundant than reactants. Under standard conditions, the PFK-1 reaction reaches equilibrium when the concentrations of all products and reactants are equal.
Many metabolites are maintained at steady-state concentrations that are far from equilibrium. A comparison of Kéq and Q, the mass-action ratio, can determine whether a metabolic reaction is far from equilibrium. The equation for this equilibrium is, fructose 6-phosphate + ATP = fructose 1,6-bisphosphate + ADP Calculate Ke, for this reaction at T = 25.0 °C. AG' = -14.2 kJ/mol Calculate the mass-action ratio, Q, from the approximate physiological concentrations for rat heart tissue shown in the table. Metabolite Concentration (µM) Q = fructose 6-phosphate 84.0 fructose 1,6-bisphosphate 25.0 АТР 12,100 ADP 1,280 Select the true statements about the PFK-1 reaction. The PFK-1 reaction in heart tissue does not reach equilibrium. In heart tissue, the PFK-1 reaction will be driven toward product formation. In heart tissue, the PFK-1 products are more abundant than reactants. Under standard conditions, the PFK-1 reaction reaches equilibrium when the concentrations of all products and reactants are equal.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 83QRT: Another step in the metabolism of glucose, which occurs after the formation of glucose6-phosphate,...
Related questions
Question
Many metabolites are maintained at steady‑state concentrations that are far from equilibrium. A comparison of ?′eq and ? , the mass‑action ratio, can determine whether a
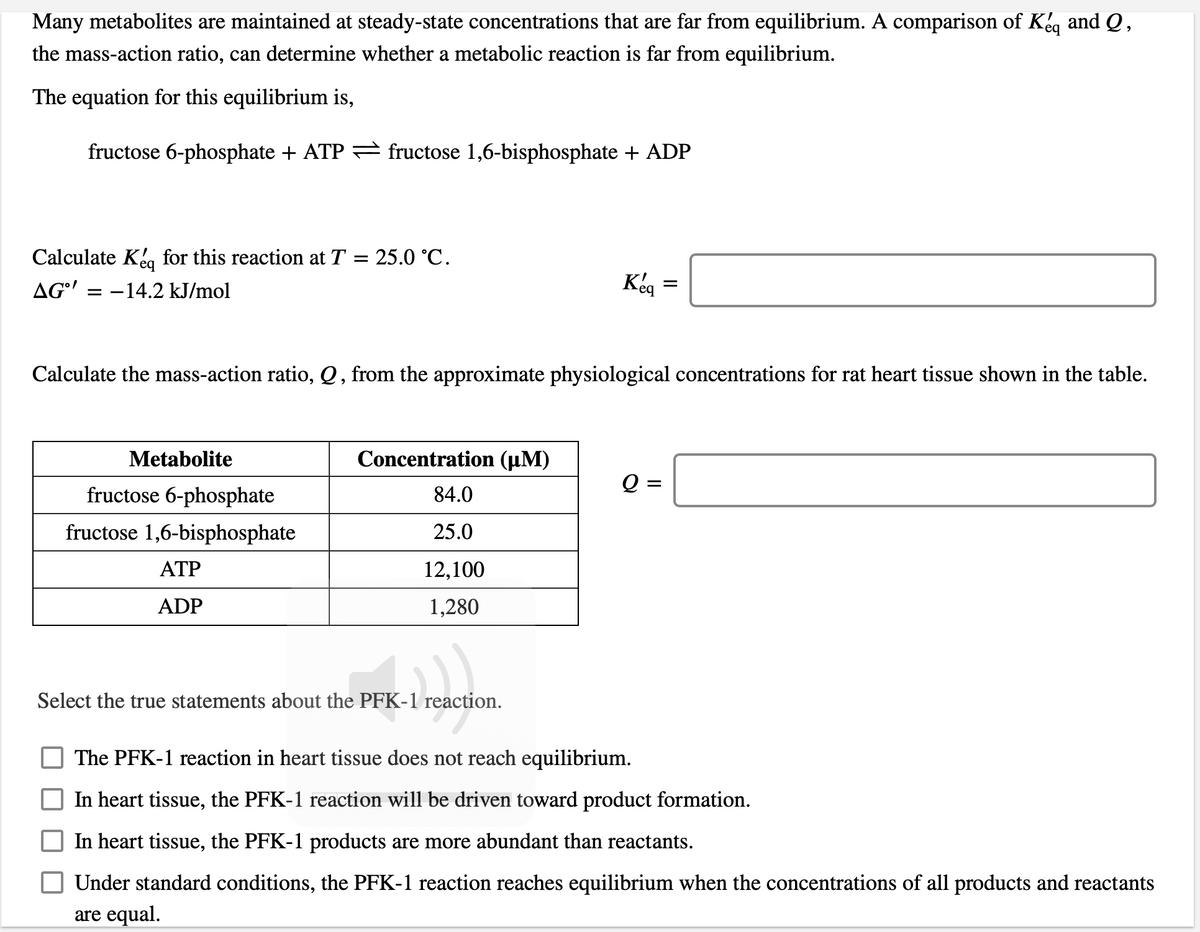
Transcribed Image Text:Many metabolites are maintained at steady-state concentrations that are far from equilibrium. A comparison of Kg and Q,
the mass-action ratio, can determine whether a metabolic reaction is far from equilibrium.
The equation for this equilibrium is,
fructose 6-phosphate + ATP = fructose 1,6-bisphosphate + ADP
Calculate Kea for this reaction at T = 25.0 °C.
AG'
-14.2 kJ/mol
Keg =
Calculate the mass-action ratio, Q , from the approximate physiological concentrations for rat heart tissue shown in the table.
Metabolite
Concentration (µM)
fructose 6-phosphate
84.0
fructose 1,6-bisphosphate
25.0
ATP
12,100
ADP
1,280
Select the true statements about the PFK-1 reaction.
The PFK-1 reaction in heart tissue does not reach equilibrium.
In heart tissue, the PFK-1 reaction will be driven toward product formation.
In heart tissue, the PFK-1 products are more abundant than reactants.
Under standard conditions, the PFK-1 reaction reaches equilibrium when the concentrations of all products and reactants
are equal.
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning