Map Roberts & Company Publishers presented by pling Leaming Organic Chenmistry Oudon For the Bronsted acid-base reaction shown below, determine the conjugate acid-base pairs. Then give the curved-arrow notation for the reaction in the left-to-right direction. (To draw the arrows, click on the reaction to get into the edit mode, then click on the curved arrow icon.) 40: 20: HaC +H H >HaC H A B C D Fill in the blarnks with the letters A,B,C, and D, representing the species in the reaction above. (It doesn't matter which pair you list first.) acid and its conjugate base acid and its conjugate base A D O Previous Give Up & View Salution)Check Answer Next Exit Hint
Map Roberts & Company Publishers presented by pling Leaming Organic Chenmistry Oudon For the Bronsted acid-base reaction shown below, determine the conjugate acid-base pairs. Then give the curved-arrow notation for the reaction in the left-to-right direction. (To draw the arrows, click on the reaction to get into the edit mode, then click on the curved arrow icon.) 40: 20: HaC +H H >HaC H A B C D Fill in the blarnks with the letters A,B,C, and D, representing the species in the reaction above. (It doesn't matter which pair you list first.) acid and its conjugate base acid and its conjugate base A D O Previous Give Up & View Salution)Check Answer Next Exit Hint
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section: Chapter Questions
Problem 4.33P: Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label...
Related questions
Question
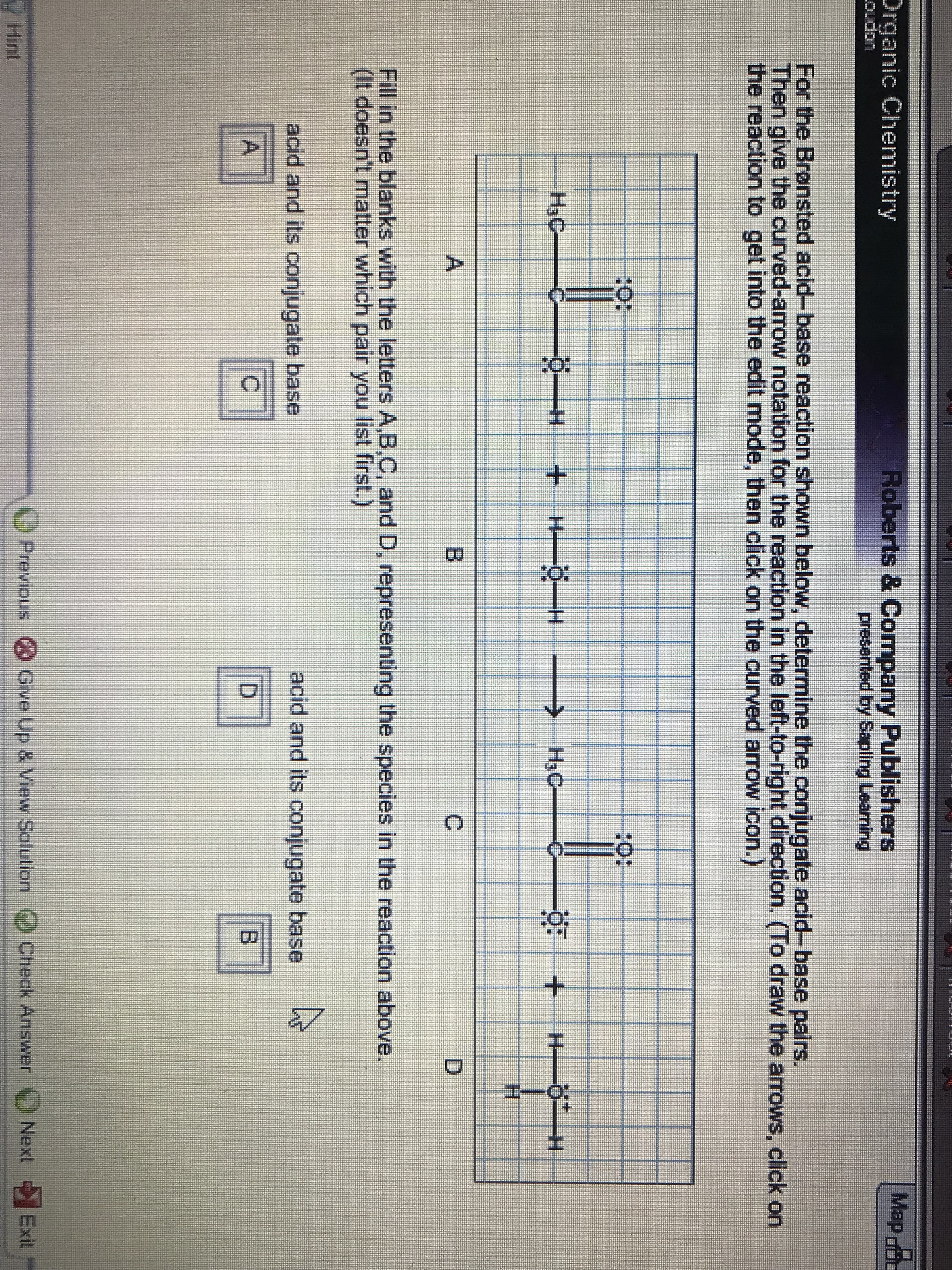
Transcribed Image Text:Map
Roberts & Company Publishers
presented by pling Leaming
Organic Chenmistry
Oudon
For the Bronsted acid-base reaction shown below, determine the conjugate acid-base pairs.
Then give the curved-arrow notation for the reaction in the left-to-right direction. (To draw the arrows, click on
the reaction to get into the edit mode, then click on the curved arrow icon.)
40:
20:
HaC
+H H
>HaC
H
A
B
C
D
Fill in the blarnks with the letters A,B,C, and D, representing the species in the reaction above.
(It doesn't matter which pair you list first.)
acid and its conjugate base
acid and its conjugate base
A
D
O Previous
Give Up & View Salution)Check Answer
Next
Exit
Hint
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning