ТАBLE 2.6 Dissociation Constants of Some Acids Acid НА A K, pK, Руruvic acid CH,COCOOH CH,C-COO 3.16 X 10-3 2.50 Formic acid НСООН HCOO 1.78 х 10-4 3.75 CH,CH–HCO0- GH;COO Lactic acid CH,CHOHCOOH 1.38 х 10-4 3.86 6.46 х 10-5 1.76 х 10-5 Веnzoic acid CH;COOH 4.19 Аcetic acid CH,COOH CH,COO 4.76 NH 5.6 х 10-10 9.25 Ammonium ion NH, Охalic acid (1) НООС—СООН НООС—СОО 5.9 х 10-2 1.23 Охalie acid (2) 6.4 х 10-3 HOOC-COO ООС—СОо 4.19 Malonic acid (1) НООС—СH,——СООН HOOC-CH,COO 1.49 х 10-3 2.83 Malonic acid (2) HOOC-CH COO НООС—СH —СНОН—СООН "OOC-CH CO 2.03 х 102-в 5.69 3.98 х 10- 5.5 х 10-6 Malic acid (1) НООС—СH,—СНОН—СОо 3.40 Malic acid (2) НООС—СH—СНОН—СОО- HOOC-CH CH,O-OOH HOOC–CH-CH,–COO OOC-CH-CHOH-CO0 5.26 Succinic acid (1) HOOC-CH-CH-COO 6.17 х 10-5 4.21 Succinic acid (2) O0C-CH-CH COO 2.3 х 10 6 5.63 Carbonic acid (1) H,CO, HCO, 4.3 x 10-7 6.37 Carbonic acid (2) HCO, Co 5.6 х 10-11 10.20 8.14 x 10 1.78 х 10-5 Citric acid (1) HOOC-CHC(OH) HOOC–CH,-C(OH) 3.09 (СООН)—СН, —соон (СООН) — СН —со- 4.75 Citric acid (2) HOOC-CH-C(OH) (COOH) OOC-CHC(OH) -CH-COO (COOH)-CH,-COO Citric acid (3) 3.9х 10-6 -00C-CH,-C(OH) (COOH) -CH-COO- -O0c-CH-C(OH) (COO")- CH-COO 5.41 Phosphoric acid (1) Phosphoric acid (2) Phosphoric acid (3) H,PO, H,PO, 7.25 х 10-3 2.14 НРО PO H,PO, 6.31 x 10- 7.20 НРО 3.98 х 10-13 12.40
ТАBLE 2.6 Dissociation Constants of Some Acids Acid НА A K, pK, Руruvic acid CH,COCOOH CH,C-COO 3.16 X 10-3 2.50 Formic acid НСООН HCOO 1.78 х 10-4 3.75 CH,CH–HCO0- GH;COO Lactic acid CH,CHOHCOOH 1.38 х 10-4 3.86 6.46 х 10-5 1.76 х 10-5 Веnzoic acid CH;COOH 4.19 Аcetic acid CH,COOH CH,COO 4.76 NH 5.6 х 10-10 9.25 Ammonium ion NH, Охalic acid (1) НООС—СООН НООС—СОО 5.9 х 10-2 1.23 Охalie acid (2) 6.4 х 10-3 HOOC-COO ООС—СОо 4.19 Malonic acid (1) НООС—СH,——СООН HOOC-CH,COO 1.49 х 10-3 2.83 Malonic acid (2) HOOC-CH COO НООС—СH —СНОН—СООН "OOC-CH CO 2.03 х 102-в 5.69 3.98 х 10- 5.5 х 10-6 Malic acid (1) НООС—СH,—СНОН—СОо 3.40 Malic acid (2) НООС—СH—СНОН—СОО- HOOC-CH CH,O-OOH HOOC–CH-CH,–COO OOC-CH-CHOH-CO0 5.26 Succinic acid (1) HOOC-CH-CH-COO 6.17 х 10-5 4.21 Succinic acid (2) O0C-CH-CH COO 2.3 х 10 6 5.63 Carbonic acid (1) H,CO, HCO, 4.3 x 10-7 6.37 Carbonic acid (2) HCO, Co 5.6 х 10-11 10.20 8.14 x 10 1.78 х 10-5 Citric acid (1) HOOC-CHC(OH) HOOC–CH,-C(OH) 3.09 (СООН)—СН, —соон (СООН) — СН —со- 4.75 Citric acid (2) HOOC-CH-C(OH) (COOH) OOC-CHC(OH) -CH-COO (COOH)-CH,-COO Citric acid (3) 3.9х 10-6 -00C-CH,-C(OH) (COOH) -CH-COO- -O0c-CH-C(OH) (COO")- CH-COO 5.41 Phosphoric acid (1) Phosphoric acid (2) Phosphoric acid (3) H,PO, H,PO, 7.25 х 10-3 2.14 НРО PO H,PO, 6.31 x 10- 7.20 НРО 3.98 х 10-13 12.40
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter6: Solutions And Colloids
Section: Chapter Questions
Problem 6.78P: 6-78 (Chemical Connections 6A) Oxides of nitrogen (NO, NO2,N2O3) are also responsible for acid rain....
Related questions
Question
Calculate the pH of a buffer solution prepared by mixing 75 mL of 1.0 M lactic acid (see Table 2.6) and 25 mL of 1.0 M sodium lactate.
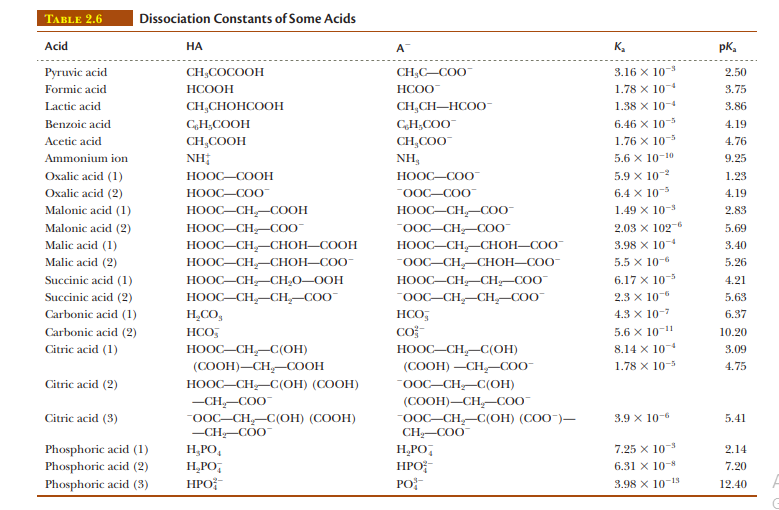
Transcribed Image Text:ТАBLE 2.6
Dissociation Constants of Some Acids
Acid
НА
A
K,
pK,
Руruvic acid
CH,COCOOH
CH,C-COO
3.16 X 10-3
2.50
Formic acid
НСООН
HCOO
1.78 х 10-4
3.75
CH,CH–HCO0-
GH;COO
Lactic acid
CH,CHOHCOOH
1.38 х 10-4
3.86
6.46 х 10-5
1.76 х 10-5
Веnzoic acid
CH;COOH
4.19
Аcetic acid
CH,COOH
CH,COO
4.76
NH
5.6 х 10-10
9.25
Ammonium ion
NH,
Охalic acid (1)
НООС—СООН
НООС—СОО
5.9 х 10-2
1.23
Охalie acid (2)
6.4 х 10-3
HOOC-COO
ООС—СОо
4.19
Malonic acid (1)
НООС—СH,——СООН
HOOC-CH,COO
1.49 х 10-3
2.83
Malonic acid (2)
HOOC-CH COO
НООС—СH —СНОН—СООН
"OOC-CH CO
2.03 х 102-в
5.69
3.98 х 10-
5.5 х 10-6
Malic acid (1)
НООС—СH,—СНОН—СОо
3.40
Malic acid (2)
НООС—СH—СНОН—СОО-
HOOC-CH CH,O-OOH
HOOC–CH-CH,–COO
OOC-CH-CHOH-CO0
5.26
Succinic acid (1)
HOOC-CH-CH-COO
6.17 х 10-5
4.21
Succinic acid (2)
O0C-CH-CH COO
2.3 х 10 6
5.63
Carbonic acid (1)
H,CO,
HCO,
4.3 x 10-7
6.37
Carbonic acid (2)
HCO,
Co
5.6 х 10-11
10.20
8.14 x 10
1.78 х 10-5
Citric acid (1)
HOOC-CHC(OH)
HOOC–CH,-C(OH)
3.09
(СООН)—СН, —соон
(СООН) — СН —со-
4.75
Citric acid (2)
HOOC-CH-C(OH) (COOH)
OOC-CHC(OH)
-CH-COO
(COOH)-CH,-COO
Citric acid (3)
3.9х 10-6
-00C-CH,-C(OH) (COOH)
-CH-COO-
-O0c-CH-C(OH) (COO")-
CH-COO
5.41
Phosphoric acid (1)
Phosphoric acid (2)
Phosphoric acid (3)
H,PO,
H,PO,
7.25 х 10-3
2.14
НРО
PO
H,PO,
6.31 x 10-
7.20
НРО
3.98 х 10-13
12.40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning