Study the acid – base reaction below and then answer the following: OH + HCO3 Reactant Side Product Side (a) draw the conjugate acid and the conjugate base products of this reaction and include all lone pairs of electrons and all formal charges (if any) (b) using the Evans pka table and the internet: I. determine in which direction this reaction will proceed and explain your reasoning I. indicate the pKa value of the acid on the reactant side and of the acid on t product side of the reaction (c) using curved arrows indicate the direction of electron flow between the two reactants on the reactant side
Study the acid – base reaction below and then answer the following: OH + HCO3 Reactant Side Product Side (a) draw the conjugate acid and the conjugate base products of this reaction and include all lone pairs of electrons and all formal charges (if any) (b) using the Evans pka table and the internet: I. determine in which direction this reaction will proceed and explain your reasoning I. indicate the pKa value of the acid on the reactant side and of the acid on t product side of the reaction (c) using curved arrows indicate the direction of electron flow between the two reactants on the reactant side
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section: Chapter Questions
Problem 4.33P: Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label...
Related questions
Question
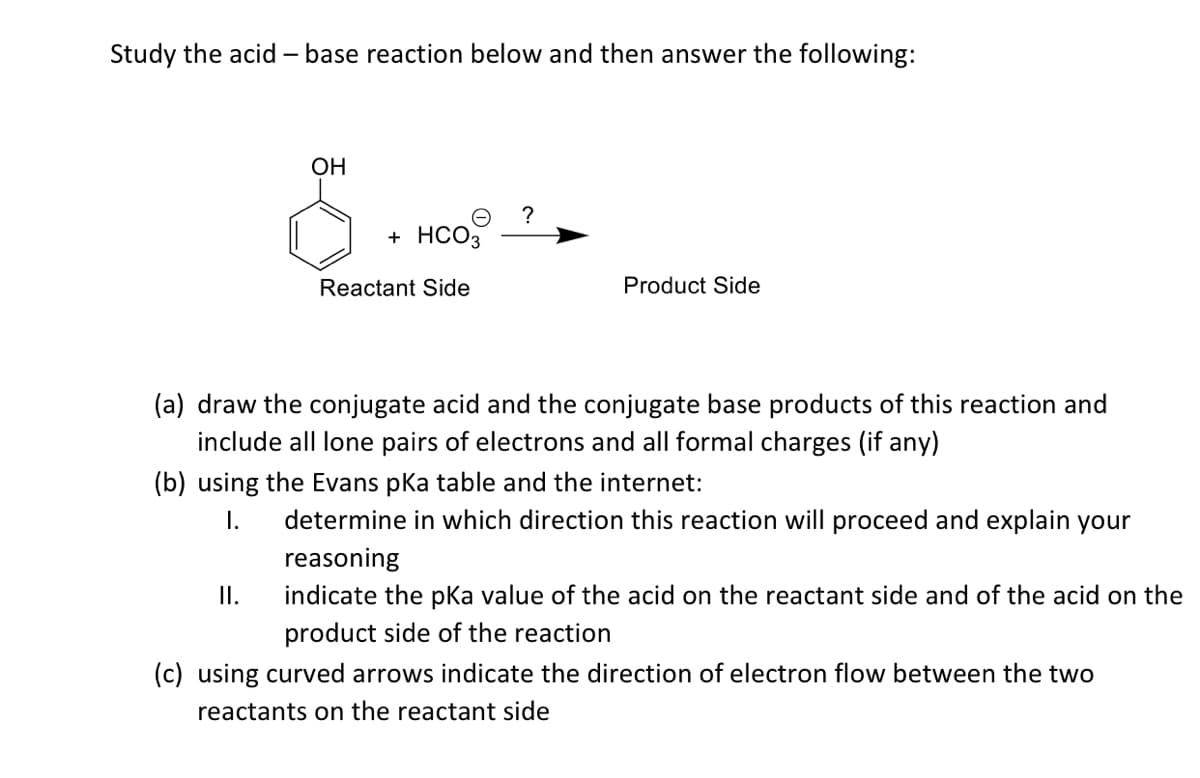
Transcribed Image Text:Study the acid – base reaction below and then answer the following:
OH
?
+ HCO3
Reactant Side
Product Side
(a) draw the conjugate acid and the conjugate base products of this reaction and
include all lone pairs of electrons and all formal charges (if any)
(b) using the Evans pka table and the internet:
I.
determine in which direction this reaction will proceed and explain your
reasoning
I.
indicate the pka value of the acid on the reactant side and of the acid on the
product side of the reaction
(c) using curved arrows indicate the direction of electron flow between the two
reactants on the reactant side
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning