Masses of compounds used and observations NaCI CusO4-5H20 Bright blue crystaline solids (NH4)2Fe(SO4)2 pale, Blue-green crystalline solid colorless Crystals Appearance of solid Mass of vial and compound (g) 12.8364 12.7398 12.9445 Mass of emptied vial (g) 11.6023 11.4888 11.9221 Mass of compound used (g) 1.2341. 1.2510. 1.0224 Volume of prepared solution = Type response here. Mass of sodium chloride used = Type response here.
Masses of compounds used and observations NaCI CusO4-5H20 Bright blue crystaline solids (NH4)2Fe(SO4)2 pale, Blue-green crystalline solid colorless Crystals Appearance of solid Mass of vial and compound (g) 12.8364 12.7398 12.9445 Mass of emptied vial (g) 11.6023 11.4888 11.9221 Mass of compound used (g) 1.2341. 1.2510. 1.0224 Volume of prepared solution = Type response here. Mass of sodium chloride used = Type response here.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 57AP
Related questions
Question
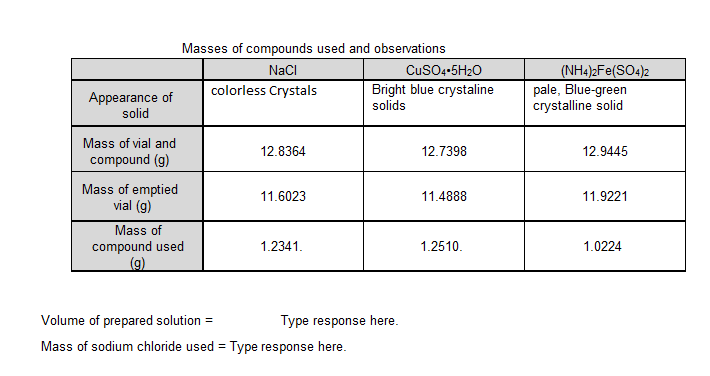
Transcribed Image Text:Masses of compounds used and observations
NaCI
CuSO4-5H20
Bright blue crystaline
solids
(NH4)2Fe(SO4)2
pale, Blue-green
crystalline solid
colorless Crystals
Appearance of
solid
Mass of vial and
12.8364
12.7398
12.9445
compound (g)
Mass of emptied
vial (g)
11.6023
11.4888
11.9221
Mass of
compound used
(g)
1.2341.
1.2510.
1.0224
Volume of prepared solution =
Type response here.
Mass of sodium chloride used = Type response here.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning