Match the letter/s that correspond to the descriptions in the given numbers: A.chemical equation AB. balanced equation AC. Chemical change AD. (aq) BD. + B.reactants BE. C.products D. coefficients CD. AE. CE. (1) E.law of conservation of matter ВС. DE. Д 81. It is the new substance formed after chemical reaction. 82. It states that atoms cannot be created nor destroyed in a chemical reaction. 83. These are used to balance an equation. 84. It indicates a forward reaction forming the products. 85. It shows that the product formed is a gas. 86. It indicates a backward reaction forming back the reactants. converts one substance to another. 87. A 88. It is an expression that uses chemical formulas and other symbols to illustrate reactants and products. 89. It is a starting material for the chemical reaction. 90. It has the same number of atoms of each element on both sides of the equations. 91. It indicates that substances are dissolved in water. 92. It indicates that the products formed are solid particles. 93. It indicates that substances used are in liquid solutions. 94. It indicates that reactions take place in the presence of heat. 95. It indicates an equilibrium reaction.
Match the letter/s that correspond to the descriptions in the given numbers: A.chemical equation AB. balanced equation AC. Chemical change AD. (aq) BD. + B.reactants BE. C.products D. coefficients CD. AE. CE. (1) E.law of conservation of matter ВС. DE. Д 81. It is the new substance formed after chemical reaction. 82. It states that atoms cannot be created nor destroyed in a chemical reaction. 83. These are used to balance an equation. 84. It indicates a forward reaction forming the products. 85. It shows that the product formed is a gas. 86. It indicates a backward reaction forming back the reactants. converts one substance to another. 87. A 88. It is an expression that uses chemical formulas and other symbols to illustrate reactants and products. 89. It is a starting material for the chemical reaction. 90. It has the same number of atoms of each element on both sides of the equations. 91. It indicates that substances are dissolved in water. 92. It indicates that the products formed are solid particles. 93. It indicates that substances used are in liquid solutions. 94. It indicates that reactions take place in the presence of heat. 95. It indicates an equilibrium reaction.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 13ALQ: What is true about the chemical properties of the product? a. The properties are more like chemical...
Related questions
Question
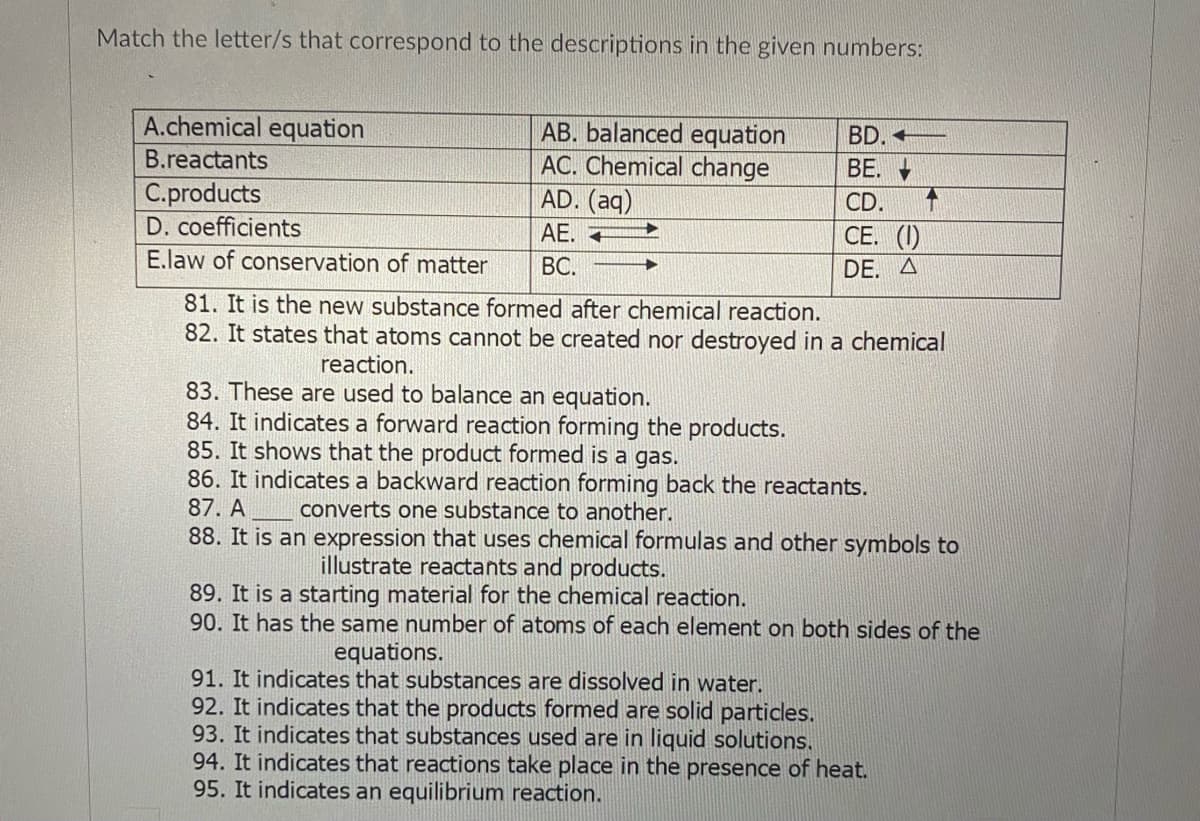
Transcribed Image Text:Match the letter/s that correspond to the descriptions in the given numbers:
A.chemical equation
AB. balanced equation
AC. Chemical change
AD. (aq)
BD. -
B.reactants
ВЕ.
C.products
D. coefficients
CD.
AE. +
СЕ. ()
E.law of conservation of matter
ВС.
DE. A
81. It is the new substance formed after chemical reaction.
82. It states that atoms cannot be created nor destroyed in a chemical
reaction.
83. These are used to balance an equation.
84. It indicates a forward reaction forming the products.
85. It shows that the product formed is a gas.
86. It indicates a backward reaction forming back the reactants.
87. A
converts one substance to another.
88. It is an expression that uses chemical formulas and other symbols to
illustrate reactants and products.
89. It is a starting material for the chemical reaction.
90. It has the same number of atoms of each element on both sides of the
equations.
91. It indicates that substances are dissolved in water.
92. It indicates that the products formed are solid particles.
93. It indicates that substances used are in liquid solutions.
94. It indicates that reactions take place in the presence of heat.
95. It indicates an equilibrium reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
