Matter is made up atoms, but in a real chemistry lab, we measure substances in grams. Therefore you need to be able to convert from grams to numbers of atoms, and vice versa. Quartz, which contains one silicon atom and two oxygen atoms per formula unit, is the second-most-common mineral on Earth after feldspar. It is used as a gemstone, as well as in pressure gauges, oscillators, resonators, wave stabilizers, heat- ray lamps, and prismastic lenses, and in the manufacture of glass, paints, abrasives, and precision instruments such as watches. Part A What is the chemical formula for quartz? Express your answer as a chemical formula. • View Available Hint(s) formula for quartz = OA chemical reaction does not occur for this question.
Matter is made up atoms, but in a real chemistry lab, we measure substances in grams. Therefore you need to be able to convert from grams to numbers of atoms, and vice versa. Quartz, which contains one silicon atom and two oxygen atoms per formula unit, is the second-most-common mineral on Earth after feldspar. It is used as a gemstone, as well as in pressure gauges, oscillators, resonators, wave stabilizers, heat- ray lamps, and prismastic lenses, and in the manufacture of glass, paints, abrasives, and precision instruments such as watches. Part A What is the chemical formula for quartz? Express your answer as a chemical formula. • View Available Hint(s) formula for quartz = OA chemical reaction does not occur for this question.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.15QAP
Related questions
Question
Please answer 16 Part A and B
Transcribed Image Text:nevie WI
abie
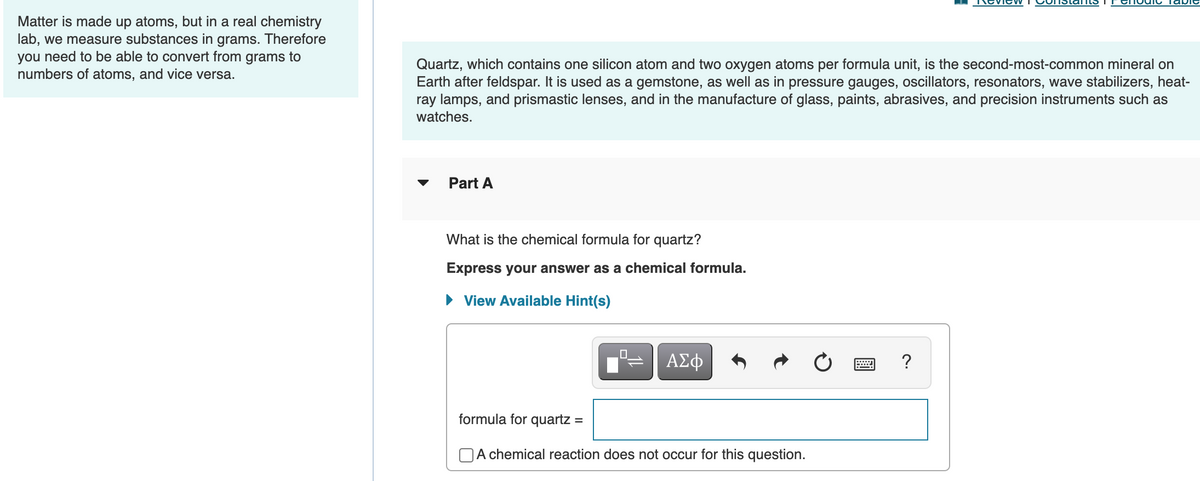
Matter is made up atoms, but in a real chemistry
lab, we measure substances in grams. Therefore
you need to be able to convert from grams to
numbers of atoms, and vice versa.
Quartz, which contains one silicon atom and two oxygen atoms per formula unit, is the second-most-common mineral on
Earth after feldspar. It is used as a gemstone, as well as in pressure gauges, oscillators, resonators, wave stabilizers, heat-
ray lamps, and prismastic lenses, and in the manufacture of glass, paints, abrasives, and precision instruments such as
watches.
Part A
What is the chemical formula for quartz?
Express your answer as a chemical formula.
View Available Hint(s)
ΑΣφ
?
formula for quartz :
OA chemical reaction does not occur for this question.
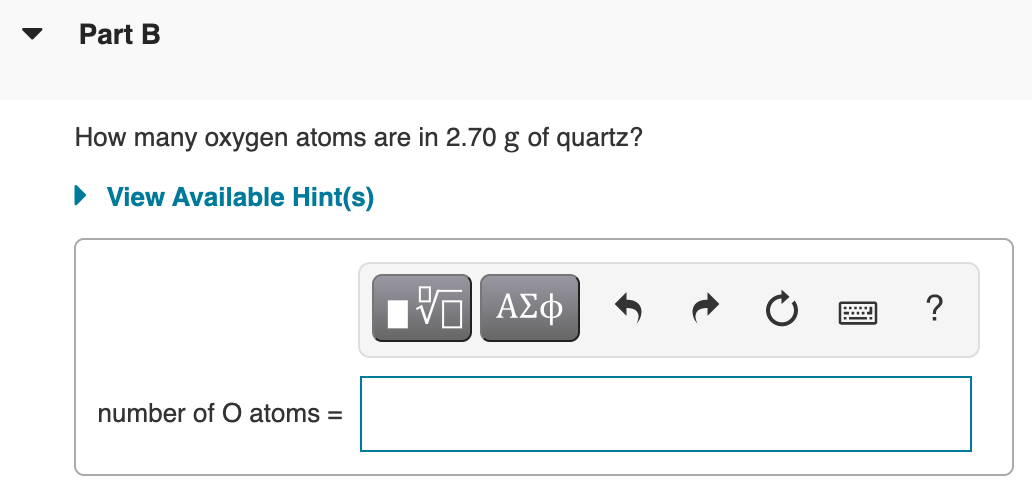
Transcribed Image Text:Part B
How many oxygen atoms are in 2.70 g of quartz?
• View Available Hint(s)
number of O atoms =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning