Chapter1: Lewis Structures
Section: Chapter Questions
Problem 63EQ
Related questions
Question
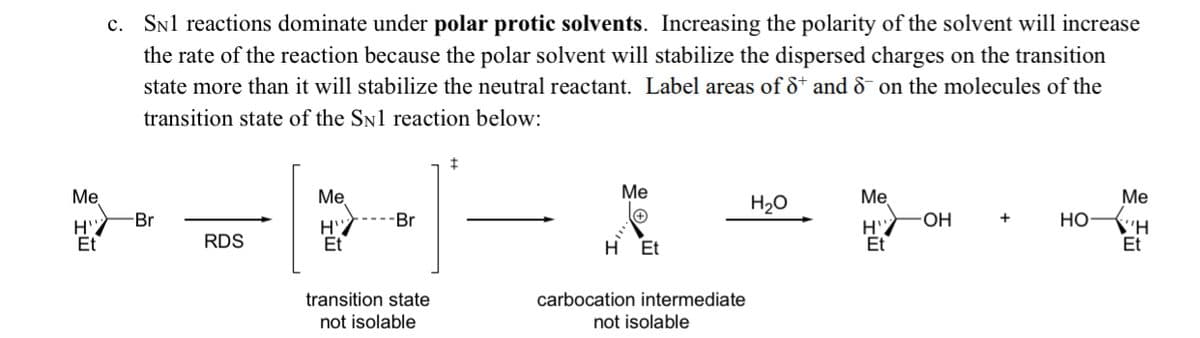
Transcribed Image Text:c. Snl reactions dominate under polar protic solvents. Increasing the polarity of the solvent will increase
the rate of the reaction because the polar solvent will stabilize the dispersed charges on the transition
state more than it will stabilize the neutral reactant. Label areas of ô* and & on the molecules of the
transition state of the SN1 reaction below:
Me
Me
Ме
Me
Me
H20
Br
-Br
OH
+
НО
\'H
Et
Et
RDS
Et
H Et
Et
transition state
not isolable
carbocation intermediate
not isolable
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning