MeO 22.55 One potential synthesis of the anti-inflammatory and analgesic drug nabumetone is chloromethylation (Problem 22.48) of 2-methoxynaphthalene followed by an acetoacetic ester synthesis (Section 19.6). 5 3 6 CH₂O CI acetoacetic ester synthesis HC 7 MeO MeO 1 8 2-Methoxynaphthalene Nabumetone (a) Account for the regioselectivity of chloromethylation at carbon 6 rather than at carbon 5 or 7. (b) Show steps in the acetoacetic ester synthesis by which the synthesis of nabum- etone is completed.
MeO 22.55 One potential synthesis of the anti-inflammatory and analgesic drug nabumetone is chloromethylation (Problem 22.48) of 2-methoxynaphthalene followed by an acetoacetic ester synthesis (Section 19.6). 5 3 6 CH₂O CI acetoacetic ester synthesis HC 7 MeO MeO 1 8 2-Methoxynaphthalene Nabumetone (a) Account for the regioselectivity of chloromethylation at carbon 6 rather than at carbon 5 or 7. (b) Show steps in the acetoacetic ester synthesis by which the synthesis of nabum- etone is completed.
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 63AP: As far back as the 16th century, South American Incas chewed the leaves of the coca bush,...
Related questions
Question
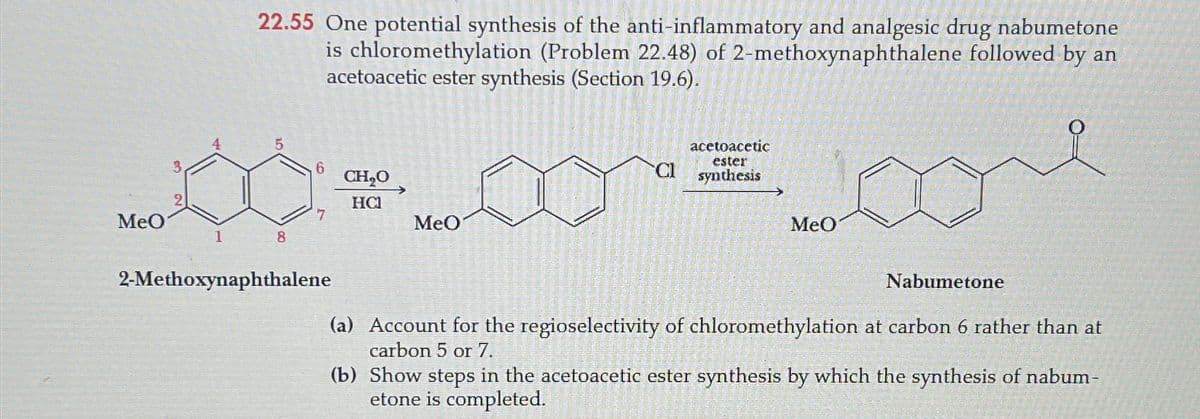
Transcribed Image Text:MeO
22.55 One potential synthesis of the anti-inflammatory and analgesic drug nabumetone
is chloromethylation (Problem 22.48) of 2-methoxynaphthalene followed by an
acetoacetic ester synthesis (Section 19.6).
5
3
6
CH₂O
CI
acetoacetic
ester
synthesis
HC
7
MeO
MeO
1
8
2-Methoxynaphthalene
Nabumetone
(a) Account for the regioselectivity of chloromethylation at carbon 6 rather than at
carbon 5 or 7.
(b) Show steps in the acetoacetic ester synthesis by which the synthesis of nabum-
etone is completed.
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning