Microbiologists use acid-alcohol when performing a cell stain of the pathogen Mycobacterium tuberculosis. A common recipe is to make 3%(wevon HCl in ethanol. HCI has a formula weight of 36.46 grams per mole. If the stock solution of HCl is 1 moles per 1000 mL, how many ml of HCI need to be added to achieve a final volume of 500 mL of acid alcohol solution? Report your answer to two decimal places. Algebra can be used to solve this problem! In this context, the percentages act as concentrations. Therefore, it is possible to use m,v; = m,v2 and use the percentages as the concentration values. We can solve for m, by converting the molarity of HCI into a percentage using its formula weight. 36.46 g HCI m, Mol HCI m = x 100 = %HCI mol HCI 1000 mL Then substitute this value of m, into the equation m1v1 = m2V2 and solve for v, m2V2 (3%) (v2) V, = 36.46 g HCI 1 mol HCI m, Mol HCI (100) 1000 mL
Microbiologists use acid-alcohol when performing a cell stain of the pathogen Mycobacterium tuberculosis. A common recipe is to make 3%(wevon HCl in ethanol. HCI has a formula weight of 36.46 grams per mole. If the stock solution of HCl is 1 moles per 1000 mL, how many ml of HCI need to be added to achieve a final volume of 500 mL of acid alcohol solution? Report your answer to two decimal places. Algebra can be used to solve this problem! In this context, the percentages act as concentrations. Therefore, it is possible to use m,v; = m,v2 and use the percentages as the concentration values. We can solve for m, by converting the molarity of HCI into a percentage using its formula weight. 36.46 g HCI m, Mol HCI m = x 100 = %HCI mol HCI 1000 mL Then substitute this value of m, into the equation m1v1 = m2V2 and solve for v, m2V2 (3%) (v2) V, = 36.46 g HCI 1 mol HCI m, Mol HCI (100) 1000 mL
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 65AP
Related questions
Question
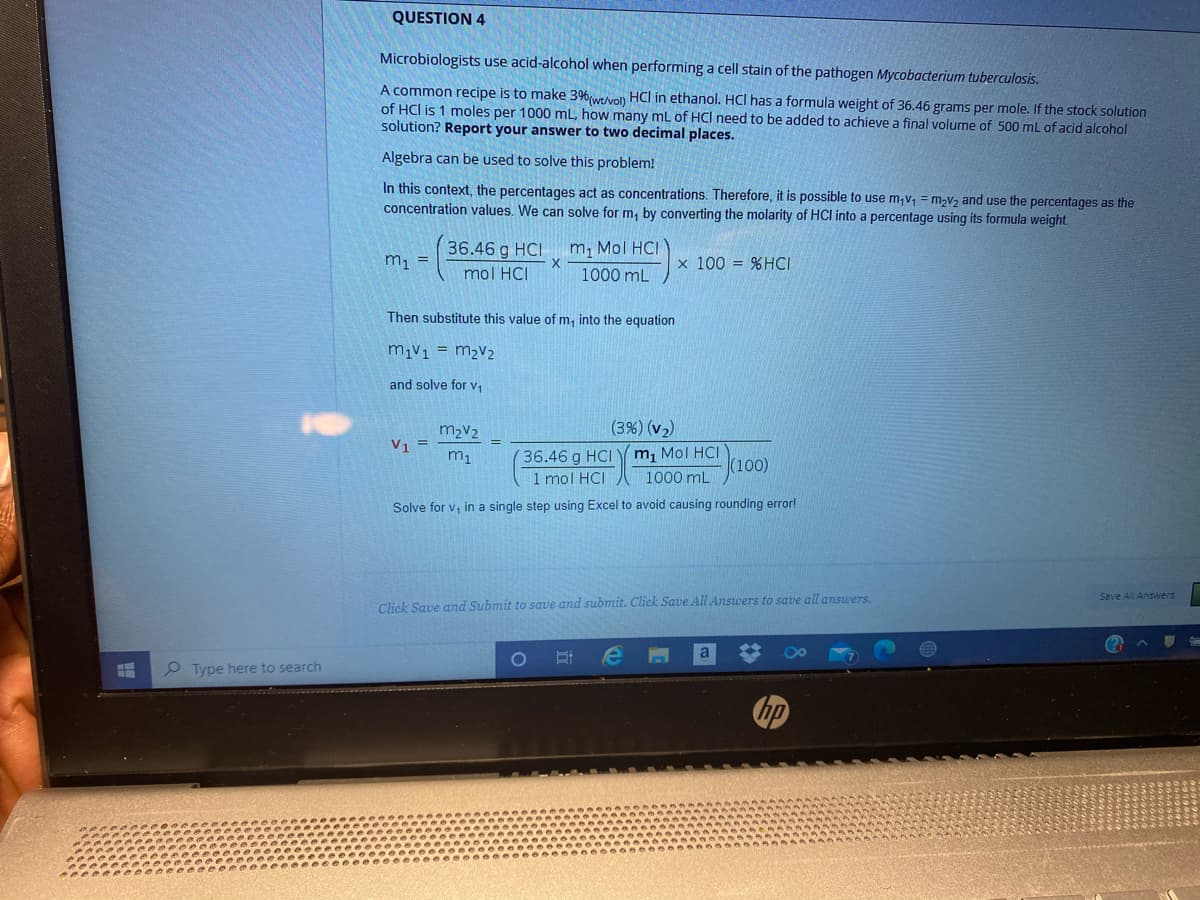
Transcribed Image Text:QUESTION 4
Microbiologists use acid-alcohol when performing a cell stain of the pathogen Mycobacterium tuberculosis.
A common recipe is to make 3%(wevo) HCI in ethanol. HCI has a formula weight of 36.46 grams per mole. If the stock solution
of HCl is 1 moles per 1000 mL, how many mL of HCI need to be added to achieve a final volume of 500 mL of acid alcohol
solution? Report your answer to two decimal places.
Algebra can be used to solve this problem!
In this context, the percentages act as concentrations. Therefore, it is possible to use m;v, = m,v2 and use the percentages as the
concentration values. We can solve for m, by converting the molarity of HCI into a percentage using its formula weight.
36.46 g HCI
m, Mol HCI
m1 =
x 100 = %HCI
mol HCI
1000 mL
Then substitute this value of m, into the equation
m1v1 = m2V2
and solve for v,
m2V2
(3%) (v2)
V1 =
36.46 g HCI Y m1 Mol HCI
1 mol HCI
(100)
1000 mL
Solve for v, in a single step using Excel to avoid causing rounding errorl
Save All Answers
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
O Type here to search
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
