MInbox (5) - bubbatx1998@gmail.cx A ALEKS Daniel Estrella - Pre-Clas X bAnswered: NAverage Bond Entha My Drive - Google Drive X X X www-awn.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IBiKFdvJdkR4Qg DFu FBLE9J HQJOYSYezxnZjy7ErUilWNytDwYCaGII7ay3s981NTPxuxnt8g-2ai_6UhWKeYIDgamBWqio?1oBw7QYjlbavbSP.... IT Welcome Aggie C... TMy Application Stat... Hazlewood Act- Te... New Tab a Amazon.com: Gene... AVeterans - Addition... T Aggie Transition Ca... StudentLoans.gov |... Apps http://bookshelf.vi... Pre-Class Quiz #17- 10/11/19 Question 1 of3 (1 point) Daniel = 2 (Na,so4) (H20) H,SOill react with solid sodium hydroxide (N2OH) to produce aqueous sodium sulfate and liquid water Aqueous sulfuric acid Suppose 52.0 g of sulfuric acid is mixed with 74. g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. dlo x10 Submit Assignment 2019 McGraw-Hill Education. All Rights Reserved Terms of Use Privacy 1:33 PM Type here to search 13 17 10/10/2019 ...
MInbox (5) - bubbatx1998@gmail.cx A ALEKS Daniel Estrella - Pre-Clas X bAnswered: NAverage Bond Entha My Drive - Google Drive X X X www-awn.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IBiKFdvJdkR4Qg DFu FBLE9J HQJOYSYezxnZjy7ErUilWNytDwYCaGII7ay3s981NTPxuxnt8g-2ai_6UhWKeYIDgamBWqio?1oBw7QYjlbavbSP.... IT Welcome Aggie C... TMy Application Stat... Hazlewood Act- Te... New Tab a Amazon.com: Gene... AVeterans - Addition... T Aggie Transition Ca... StudentLoans.gov |... Apps http://bookshelf.vi... Pre-Class Quiz #17- 10/11/19 Question 1 of3 (1 point) Daniel = 2 (Na,so4) (H20) H,SOill react with solid sodium hydroxide (N2OH) to produce aqueous sodium sulfate and liquid water Aqueous sulfuric acid Suppose 52.0 g of sulfuric acid is mixed with 74. g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. dlo x10 Submit Assignment 2019 McGraw-Hill Education. All Rights Reserved Terms of Use Privacy 1:33 PM Type here to search 13 17 10/10/2019 ...
Chapter31: Synthetic Polymers
Section31.SE: Something Extra
Problem 21AP
Related questions
Question
Transcribed Image Text:MInbox (5) - bubbatx1998@gmail.cx
A ALEKS Daniel Estrella - Pre-Clas X
bAnswered: NAverage Bond Entha
My Drive - Google Drive
X
X
X
www-awn.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IBiKFdvJdkR4Qg DFu FBLE9J HQJOYSYezxnZjy7ErUilWNytDwYCaGII7ay3s981NTPxuxnt8g-2ai_6UhWKeYIDgamBWqio?1oBw7QYjlbavbSP....
IT Welcome Aggie C...
TMy Application Stat...
Hazlewood Act- Te...
New Tab
a Amazon.com: Gene...
AVeterans - Addition...
T Aggie Transition Ca...
StudentLoans.gov |...
Apps
http://bookshelf.vi...
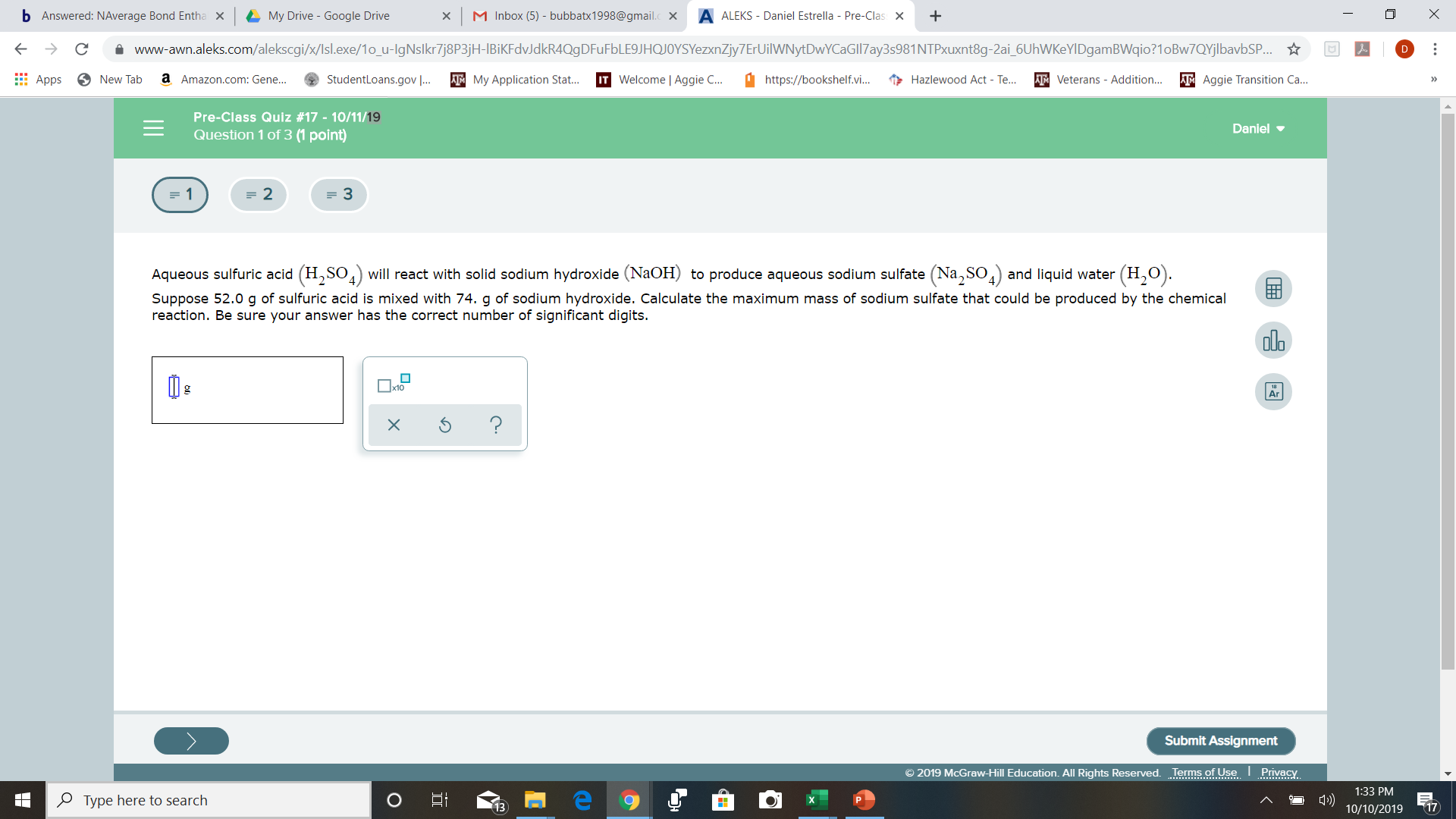
Pre-Class Quiz #17- 10/11/19
Question 1 of3 (1 point)
Daniel
= 2
(Na,so4)
(H20)
H,SOill react with solid sodium hydroxide (N2OH) to produce aqueous sodium sulfate
and liquid water
Aqueous sulfuric acid
Suppose 52.0 g of sulfuric acid is mixed with 74. g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical
reaction. Be sure your answer has the correct number of significant digits.
dlo
x10
Submit Assignment
2019 McGraw-Hill Education. All Rights Reserved
Terms of Use
Privacy
1:33 PM
Type here to search
13
17
10/10/2019
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps with 3 images

Recommended textbooks for you

