Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter17: Acid-base(proton Transfer) Reactions
Section: Chapter Questions
Problem 8E: Aluminum chloride, AlCl3, behaves more as a molecular compound than an ionic one. This is...
Related questions
Question
100%
Help 7
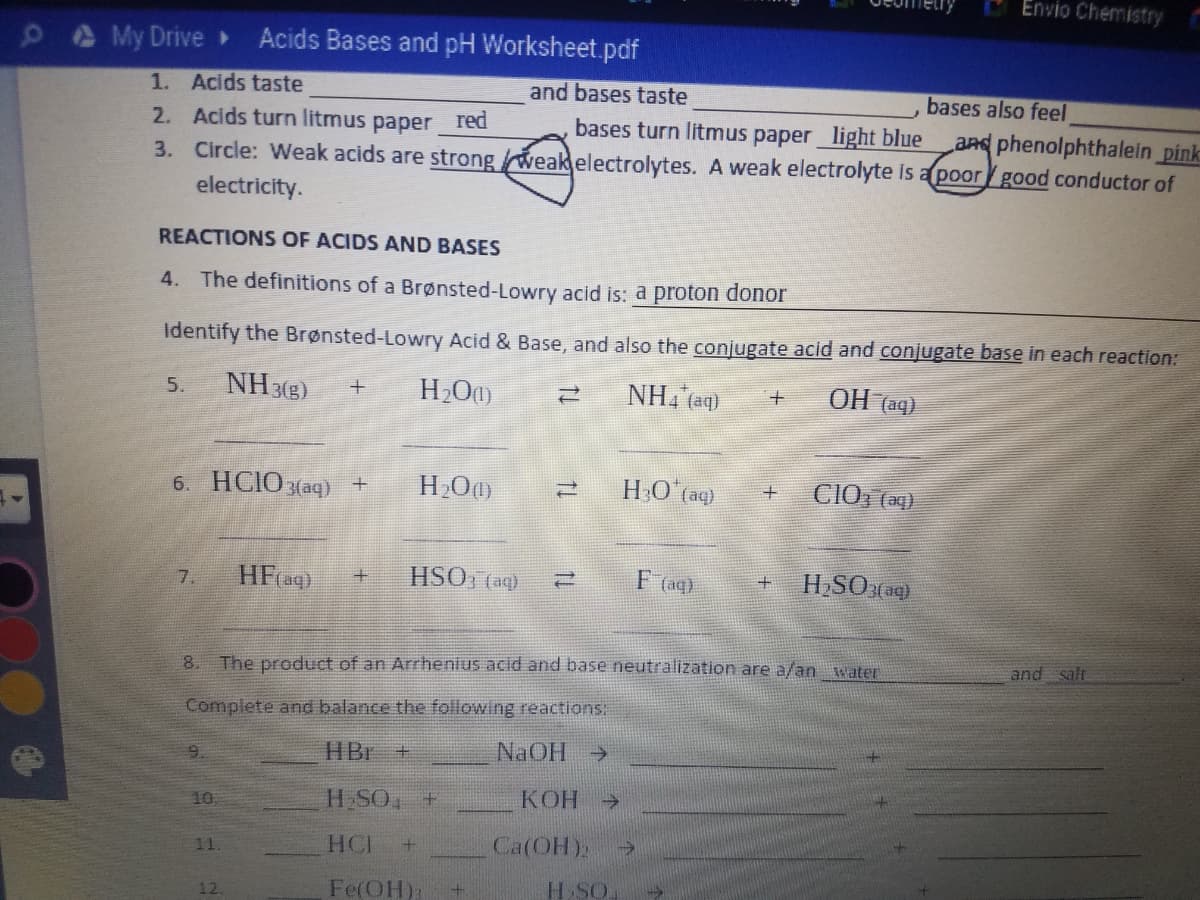
Transcribed Image Text:Envio Chemistry
My Drive»
Acids Bases and pH Worksheet.pdf
1. Acids taste
2. Acids turn litmus paper red
3. Circle: Weak acids are strongweakelectrolytes. A weak electrolyte is a poorgood conductor of
and bases taste
bases also feel
bases turn litmus paper light blue
aRd phenolphthalein pink
electricity.
REACTIONS OF ACIDS AND BASES
4. The definitions of a Brønsted-Lowry acid is: a proton donor
Identify the Brønsted-Lowry Acid & Base, and also the conjugate acid and conjugate base in each reaction:
NH 3(3)
NH, (aq)
OH (aq)
5.
6. HCIOзаq) +
HO aq)
CIO, (ag)
HFag)
HSO, (aq)
F (ag)
HSO(aq)
7.
and salt
8.
The product of an Arrhenius acid and base neutralization are a/an water
Complete and balance the following reactions:
HBr +
NaOH >
9.
10
H SO, +
КОН
->
HCI
Ca(OH):
11.
Fe(OH):
H SO
12.
Expert Solution
Step 1
This is an example of acid base reaction.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning