MISSED THIS? Read Section 18.2 (Pages 788-799): Watch KCV 18.28. IWE 18.2. Solve an equilibrium problem (using an E table) to calculate the pH of each of the following solutions. com/nyct/itentViewTassignment ProblemiD-200959836 Part A 0.17 M CH3NH₂ (where K₁, for CH₂NH₂ is 4.4 x 10) Express your answer using two decimal places. 15. ΑΣΦ PH Submit Part B Request Answer 0.17 M CHÍNH,CH Express your answer using two decimal places. VE ΑΣΦΑ pH =
MISSED THIS? Read Section 18.2 (Pages 788-799): Watch KCV 18.28. IWE 18.2. Solve an equilibrium problem (using an E table) to calculate the pH of each of the following solutions. com/nyct/itentViewTassignment ProblemiD-200959836 Part A 0.17 M CH3NH₂ (where K₁, for CH₂NH₂ is 4.4 x 10) Express your answer using two decimal places. 15. ΑΣΦ PH Submit Part B Request Answer 0.17 M CHÍNH,CH Express your answer using two decimal places. VE ΑΣΦΑ pH =
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 127QRT
Related questions
Question
Please provide only typed answer solution no handwritten solution needed allowed
Please do it all instant positive rating
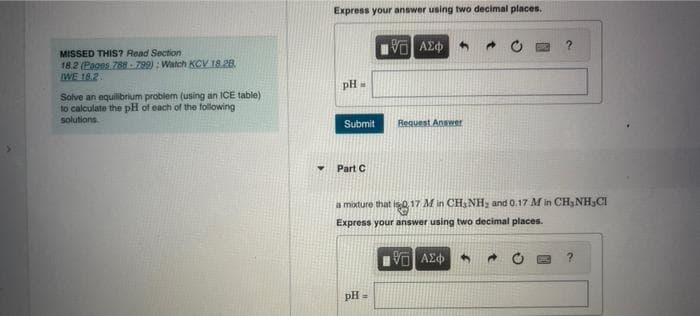
Transcribed Image Text:MISSED THIS? Read Section
18.2 (Pages 788-799): Watch KCV 18.28.
IWE 18.2.
Solve an equilibrium problem (using an ICE table)
to calculate the pH of each of the following
solutions.
Express your answer using two decimal places.
PH
M
Submit
Part C
ΑΣΦ
pH =
Request Answer
a mixture that is 0.17 M in CH₂NH₂ and 0.17 M in CH₂NH,CI
Express your answer using two decimal places.
VO ΑΣΦ
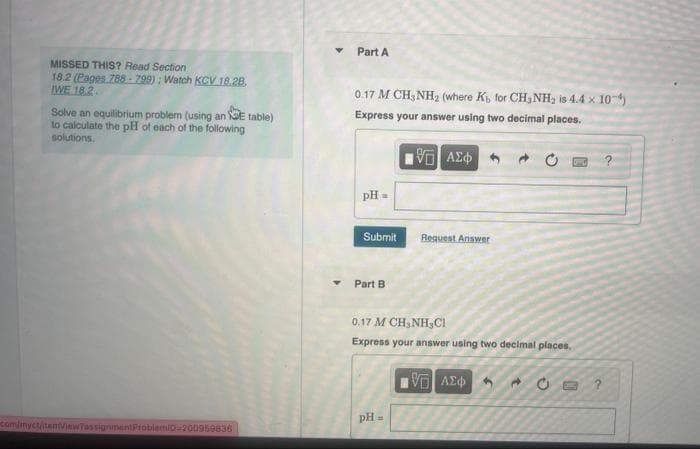
Transcribed Image Text:MISSED THIS? Read Section
18.2 (Pages 788-799): Watch KCV 18.28.
WE 18.2.
Solve an equilibrium problem (using an E table)
to calculate the pH of each of the following
solutions.
com/myct/item/viewTassignment ProblemiD-200959836
Y
Part A
0.17 M CH3NH₂ (where K, for CH₂NH₂ is 4.4 x 10-¹)
Express your answer using two decimal places.
15. ΑΣΦ
pH =
Submit
Part B
Request Answer
0.17 M CH,NH,C1
Express your answer using two decimal places.
pH =
IVE ΑΣΦΑ
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning