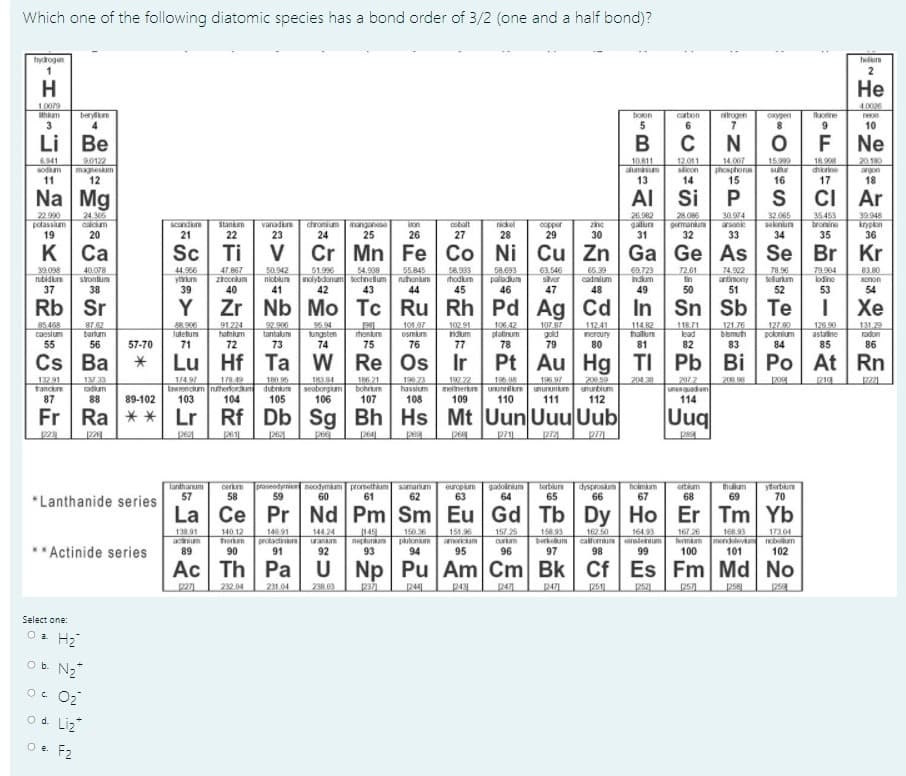
十 Mo TeRuR/P. Which one of the following diatomic species has a bond order of 3/2 (one and a half bond)? hydrogen H Не 4.0006 10079 thm berylm boon carbon nirogen oxypen uorine 3 6. 9. 10 Li Be F Ne 90122 10 811 aluminm 13 18.90 6941 sodium 12.011 slcon 14 14.007 phosphorus 15 15999 magneskam 12 20.180 argon 18 chorine 11 16 17 Na Mg AI Si 22.990 potassium 19 32.065 seknun 2692 28.086 germanium 30.974 arsanie 24.306 39.948 35453 tromine vanad 23 nickel 28 lankm chromium manganese 24 25 scandun on cobalt zine copper 29 20 26 27 30 31 33 34 35 36 к Са Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 40.078 51.996 nicblum nolybdanum bechnetium 42 58.83 hodkum 79 96 tellurkum 52 83.80 39.038 rubidurn 47.867 50.942 54.938 55.845 nuhonum 58.093 palladium 46 63.546 sver 65.39 cadmium CA.723 74.902 79.904 lodine Xnon 37 38 40 41 43 44 45 47 48 49 51 53 54 Rb Sr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Хе 85 468 caesium 55 106.42 8762 barum 56 5.94 ungsten 102 91 92.900 tantalum 73 101.07 osmum 107 87 gold 79 112.41 114.82 halum 81 118.71 lead 121.76 bismuh 83 127.80 poknium 126.90 astaine 131.29 radon henkum neroury 57-70 74 75 76 77 78 80 82 84 85 86 Cs Lu Hf Ta w Re Pt Au Hg TI Pb Bi Po At Rn | Ро 13 33 adium TR6 21 bohrum 107 190 21 hassium 132.91 trance 184 seaborgam 106 196 08 melinertum Uunnilm unununium 110 197 72 204 38 20 8 p221 180 95 dubnium 105 200.59 ununbium 112 2012 unenquadum 114 87 88 89-102 108 109 111 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq pa 264 Tanthanum 57 neodymium promethaum samariam 61 europium 63 gadoinum 64 terbiurn 65 dysproskam holmm 66 67 ortm 68 ytertun 70 thuum *Lanthanide series 58 59 60 62 69 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 140 91 protadinu anum 91 157 25 curam 96 164 93 140.12 unEe horkm 90 138.91 150.36 t68.93 Termam mendlevm 100 144.24 167 26 149 nepkuram 93 151.96 158.93 Derkum 97 162 50 17304 Ium 102 Actinide series 89 92 94 95 98 99 101 Ac Th Pa Np Pu Am Cm Bk Cf Es Fm Md No 232 04 231 04 238 03 2371 244 247 2471 251 Select one: O a H2 Ob N2 O. O2 od Liz Oe F2 の Y 3 与
Formal Charges
Formal charges have an important role in organic chemistry since this concept helps us to know whether an atom in a molecule is neutral/bears a positive or negative charge. Even if some molecules are neutral, the atoms within that molecule need not be neutral atoms.
Polarity Of Water
In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. As oxygen is more electronegative than hydrogen thus, there exists polarity in the bonds which is why water is known as a polar solvent.
Valence Bond Theory Vbt
Valence bond theory (VBT) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. It gives a quantum mechanical approach to the formation of covalent bonds with the help of wavefunctions using attractive and repulsive energies when two atoms are brought from infinity to their internuclear distance.
Step by step
Solved in 3 steps with 3 images









