Molar Mass of Vitamin C 40 M Trial Grams of Initial Final mL of NaOH Number Vitamin C buret Reading Reading buret used in titration Weighed 1 2.569 0.70 23.86 23.16 2.690 6.75 32.50 25.75 CALCULATIONS 2.
Molar Mass of Vitamin C 40 M Trial Grams of Initial Final mL of NaOH Number Vitamin C buret Reading Reading buret used in titration Weighed 1 2.569 0.70 23.86 23.16 2.690 6.75 32.50 25.75 CALCULATIONS 2.
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
100%
Calculate the number of moles of vitamin C in the tablet based on the stoichimetry from the equation given in the back ground
1000 mg is in each tablet
Transcribed Image Text:Editin
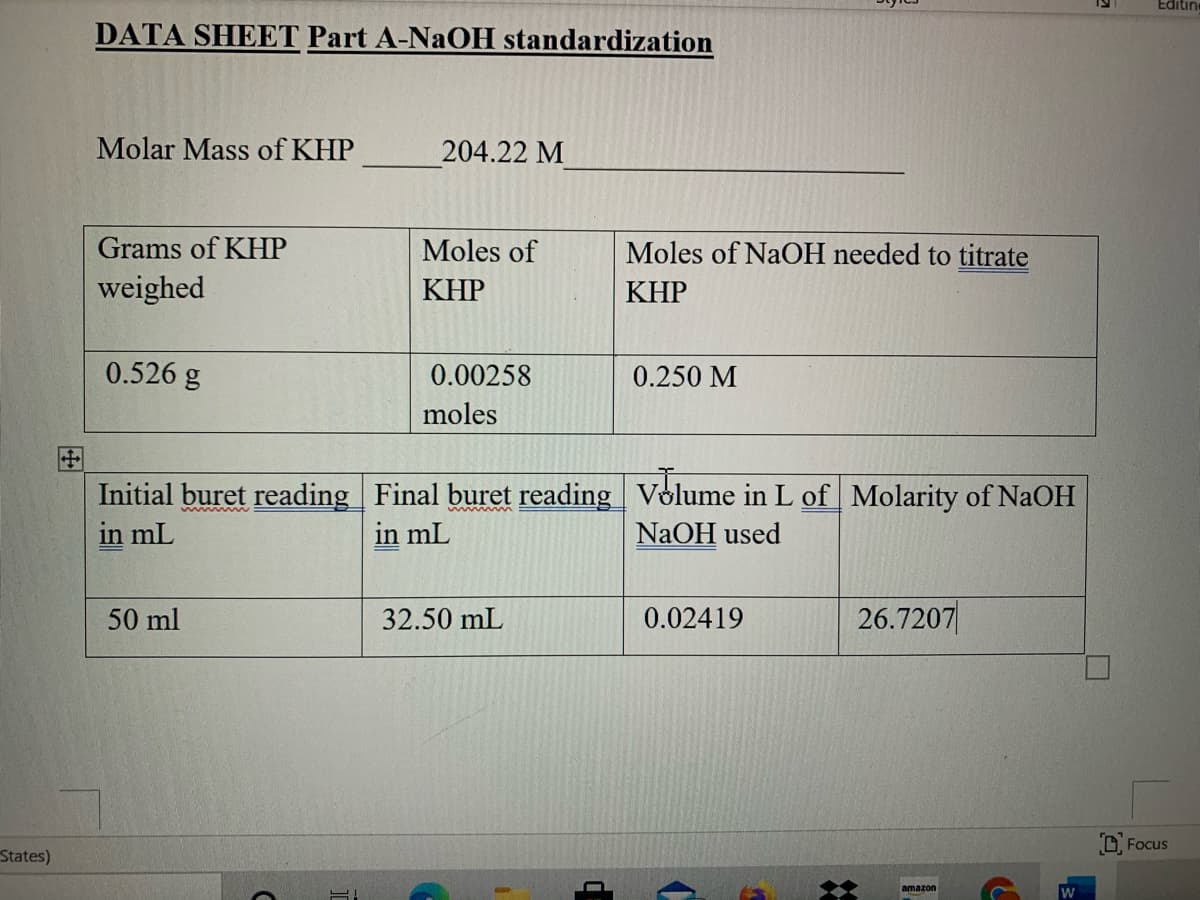
DATA SHEET Part A-NaOH standardization
Molar Mass of KHP
204.22 M
Grams of KHP
Moles of
Moles of NaOH needed to titrate
weighed
KHP
KHP
0.526 g
0.00258
0.250 M
moles
Initial buret reading Final buret reading Volume in L of Molarity of NaOH
in mL
in mL
NAOH used
50 ml
32.50 mL
0.02419
26.7207
D Focus
States)
amazon
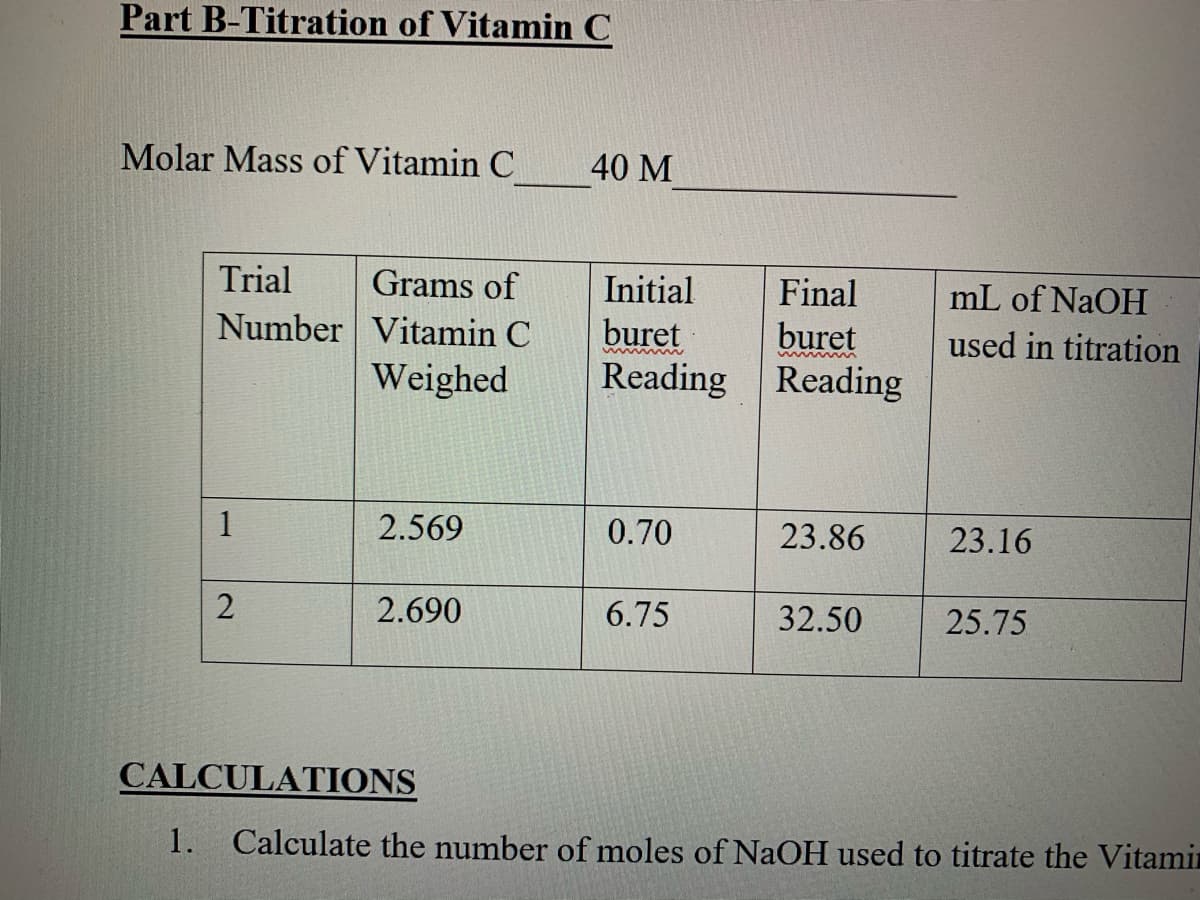
Transcribed Image Text:Part B-Titration of Vitamin C
Molar Mass of Vitamin C
40 M
Trial
Grams of
Initial
buret
Reading Reading
Final
mL of NaOH
Number Vitamin C
Weighed
buret
used in titration
1
2.569
0.70
23.86
23.16
2.690
6.75
32.50
25.75
CALCULATIONS
1.
Calculate the number of moles of NaOH used to titrate the Vitamin
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole