Molecules that do not these substances are covalently bonded contrast, a(n) Electrolytes include salts, acids, bases, and some electrolyte refers directly to the ability of these substances, when dissolved and dissociated in solution, to (or come apart) in solution are called Most of (e.g.glucose, urea, and creatinine). In is any substance that dissociates in solution to form charged proteins. The term
Molecules that do not these substances are covalently bonded contrast, a(n) Electrolytes include salts, acids, bases, and some electrolyte refers directly to the ability of these substances, when dissolved and dissociated in solution, to (or come apart) in solution are called Most of (e.g.glucose, urea, and creatinine). In is any substance that dissociates in solution to form charged proteins. The term
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.12QAP
Related questions
Question
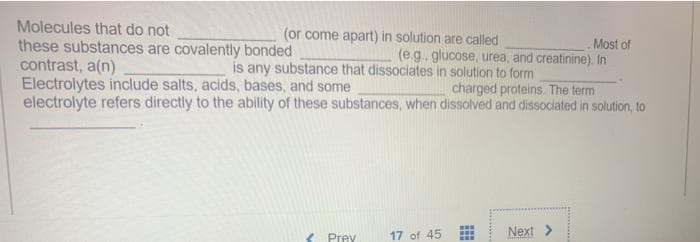
Transcribed Image Text:Molecules that do not
these substances are covalently bonded
contrast, a(n)
Electrolytes include salts, acids, bases, and some
electrolyte refers directly to the ability of these substances, when dissolved and dissociated in solution, to
(or come apart) in solution are called
Most of
(e.g.. glucose, urea, and creatinine). In
is any substance that dissociates in solution to form
charged proteins. The term
17 of 45
Next >
Prev
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,