MULTIPLE CHOICE. Read and understand the questions below. Choose the letter of your answer 1. Which of the following graphs explains the relationship of vapor and temperature? А. С. В. D. None of these 2. Which property of matter shows a liquid's resistance to flow? С. Ductility Viscosity Boiling point Being brittle А. В. D. 3. Why is that water has a higher surface tension that alcohol? A. Water has stronger intermolecular forces of attraction than alcohol. B. Water has lower intermolecular forces of attraction than alcohol. C. Water is in liquid phase while alcohol is in gas phase. D. All of the above. 4. Which of the following situations tells us that heat of vaporization is observed? A. An insect floats on the surface of the water. B. An athlete feels cool after sweating profusely. C. Water turned to ice after being placed in the freezer. D. A child hears a popping sound when a bottle of soft drink is open. 5. Which temperature has the lowest intermolecular forces of attraction? А. 0°С В. 25°С C. 50°C D. 100°C B.
MULTIPLE CHOICE. Read and understand the questions below. Choose the letter of your answer 1. Which of the following graphs explains the relationship of vapor and temperature? А. С. В. D. None of these 2. Which property of matter shows a liquid's resistance to flow? С. Ductility Viscosity Boiling point Being brittle А. В. D. 3. Why is that water has a higher surface tension that alcohol? A. Water has stronger intermolecular forces of attraction than alcohol. B. Water has lower intermolecular forces of attraction than alcohol. C. Water is in liquid phase while alcohol is in gas phase. D. All of the above. 4. Which of the following situations tells us that heat of vaporization is observed? A. An insect floats on the surface of the water. B. An athlete feels cool after sweating profusely. C. Water turned to ice after being placed in the freezer. D. A child hears a popping sound when a bottle of soft drink is open. 5. Which temperature has the lowest intermolecular forces of attraction? А. 0°С В. 25°С C. 50°C D. 100°C B.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 68QAP: In the blanks provided, answer the questions below, using LT (for is less than), GT (for is greater...
Related questions
Question
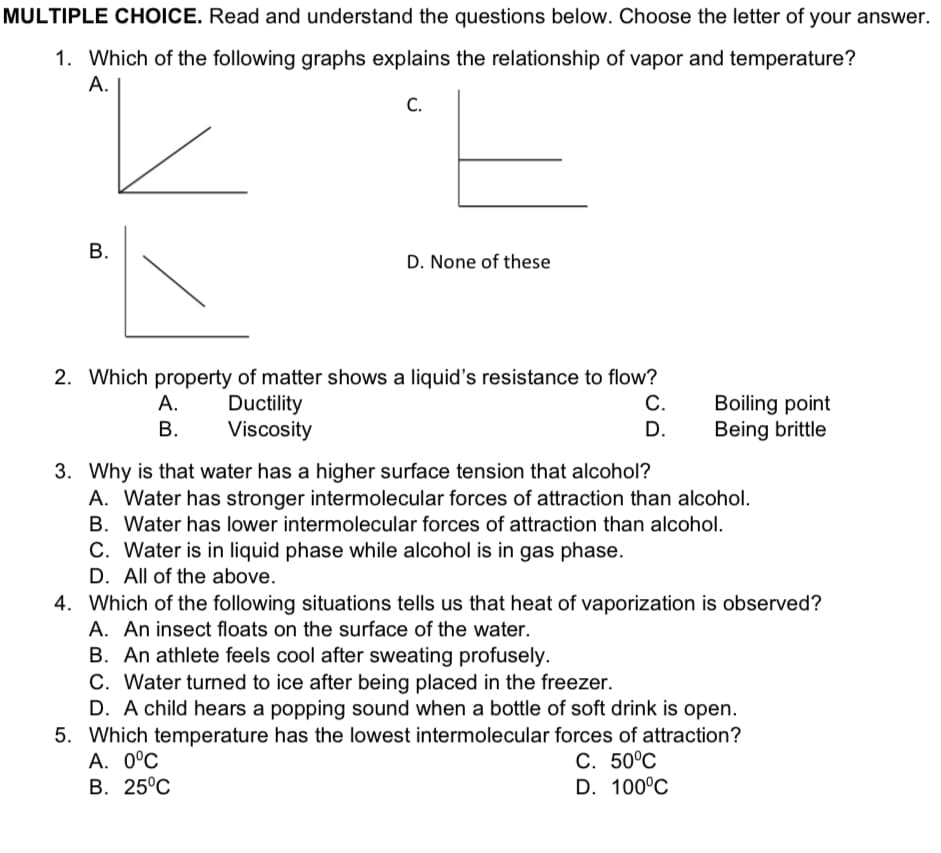
Transcribed Image Text:MULTIPLE CHOICE. Read and understand the questions below. Choose the letter of your answer.
1. Which of the following graphs explains the relationship of vapor and temperature?
A.
C.
D. None of these
2. Which property of matter shows a liquid's resistance to flow?
C.
Boiling point
Being brittle
А.
Ductility
Viscosity
С.
В.
D.
3. Why is that water has a higher surface tension that alcohol?
A. Water has stronger intermolecular forces of attraction than alcohol.
B. Water has lower intermolecular forces of attraction than alcohol.
C. Water is in liquid phase while alcohol is in gas phase.
D. All of the above.
4. Which of the following situations tells us that heat of vaporization is observed?
A. An insect floats on the surface of the water.
B. An athlete feels cool after sweating profusely.
C. Water turned to ice after being placed in the freezer.
D. A child hears a popping sound when a bottle of soft drink is open.
5. Which temperature has the lowest intermolecular forces of attraction?
A. 0°C
B. 25°C
С. 50°С
D. 100°C
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning