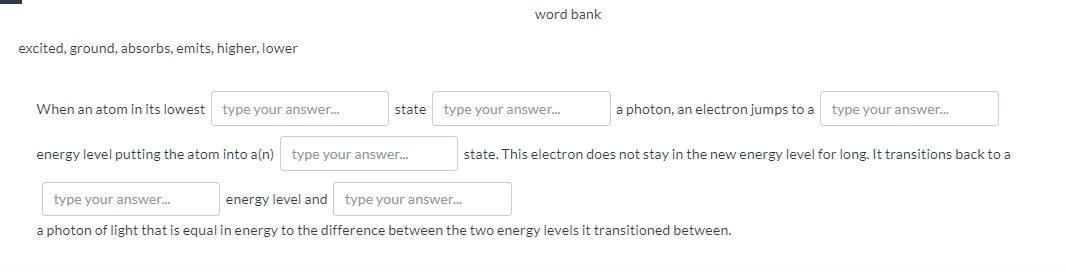
nan atom in its lowest type your answer. state type your answer. a photon, an electron jumps to a type your answer. gy level putting the atom into a(n) type your answer. state. This electron does not stay in the new energy level for long. It transitions back to a pe your answer. energy level and type your answer. ton of light that is equal in energy to the difference between the two energy levels it transitioned between.
nan atom in its lowest type your answer. state type your answer. a photon, an electron jumps to a type your answer. gy level putting the atom into a(n) type your answer. state. This electron does not stay in the new energy level for long. It transitions back to a pe your answer. energy level and type your answer. ton of light that is equal in energy to the difference between the two energy levels it transitioned between.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter5: Electrons In The Atom
Section5.3: Electron Configuration
Problem 22PP
Related questions
Question
Transcribed Image Text:word bank
excited, ground, absorbs, emits, higher, lower
When an atom in its lowest
type your answer.
state
type your answer.
a photon, an electron jumps to a
type your answer.
energy level putting the atom into a(n)
type your answer.
state. This electron does not stay in the new energy level for long. It transitions back to a
type your answer.
energy level and
type your answer.
a photon of light that is equal in energy to the difference between the two energy levels it transitioned between.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
