This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: B energy A Use this diagram to complete the table below. Which is the ground state? A How many excited states are there? 2 How many lines are in the absorption line spectrum? 3 Which transition causes the emission line at the shortest wavelength? Which transition causes the emission line at the longest wavelength? → ||
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: B energy A Use this diagram to complete the table below. Which is the ground state? A How many excited states are there? 2 How many lines are in the absorption line spectrum? 3 Which transition causes the emission line at the shortest wavelength? Which transition causes the emission line at the longest wavelength? → ||
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 44AP: The energy needed to ionize an atom of element X when it is in its most stable state is 500kJmol1 ....
Related questions
Question
100%
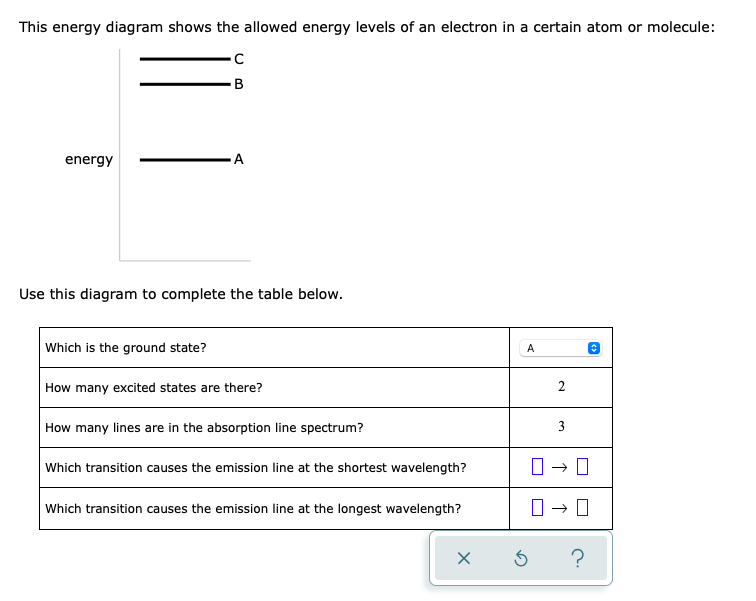
Transcribed Image Text:This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule:
-B
energy
-A
Use this diagram to complete the table below.
Which is the ground state?
A
How many excited states are there?
2
How many lines are in the absorption line spectrum?
3
Which transition causes the emission line at the shortest wavelength?
Which transition causes the emission line at the longest wavelength?
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,