NDTAP Q Search this course tions Use the References to access important values if needed for this question. The water in a pressure cooker boils at a temperature greater than 100°C because it is under pressure. At this higher temperature, the chemical reactions associated with the cooking of food take place at a greater rate. (a) Some food cooks fully in 6.00 min in a pressure cooker at 113.0°C and in 30.0 minutes in an open pot at 100.0°C. Calculate the average activation energy reactions associated with the cooking of this food. kJ mol1 food take to cook in an open pot of boiling water at an altitude of 5000 feet, where the boiling point of water is 94.9 C? min Submit Answer 3 question attempts remaining Autosaved at 9:31 AM Nex Back 9:31 AM
NDTAP Q Search this course tions Use the References to access important values if needed for this question. The water in a pressure cooker boils at a temperature greater than 100°C because it is under pressure. At this higher temperature, the chemical reactions associated with the cooking of food take place at a greater rate. (a) Some food cooks fully in 6.00 min in a pressure cooker at 113.0°C and in 30.0 minutes in an open pot at 100.0°C. Calculate the average activation energy reactions associated with the cooking of this food. kJ mol1 food take to cook in an open pot of boiling water at an altitude of 5000 feet, where the boiling point of water is 94.9 C? min Submit Answer 3 question attempts remaining Autosaved at 9:31 AM Nex Back 9:31 AM
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 65SCQ
Related questions
Question
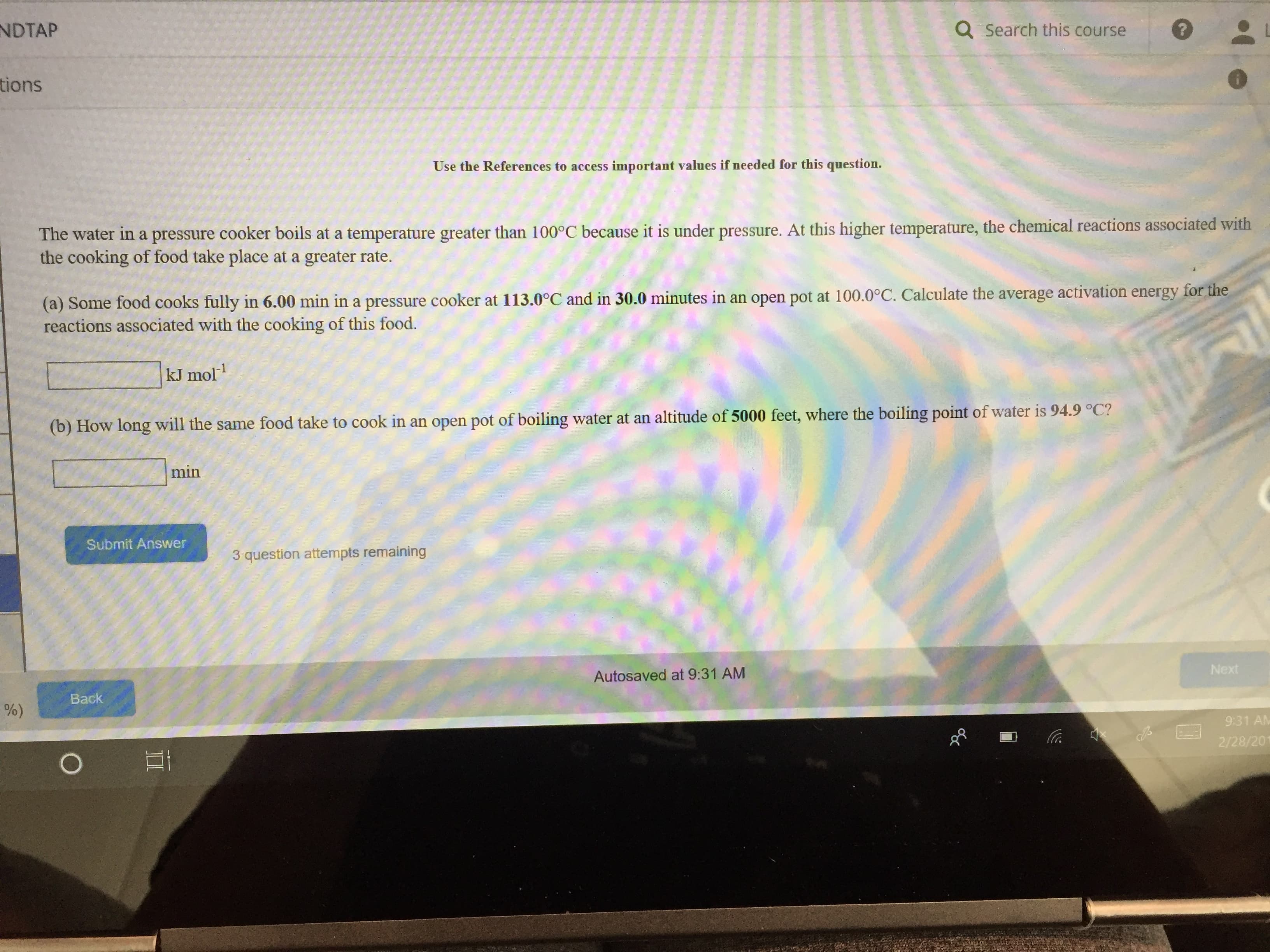
Transcribed Image Text:NDTAP
Q Search this course
tions
Use the References to access important values if needed for this question.
The water in a pressure cooker boils at a temperature greater than 100°C because it is under pressure. At this higher temperature, the chemical reactions associated with
the cooking of food take place at a greater rate.
(a) Some food cooks fully in 6.00 min in a pressure cooker at 113.0°C and in 30.0 minutes in an open pot at 100.0°C. Calculate the average activation energy
reactions associated with the cooking of this food.
kJ mol1
food take to cook in an open pot of boiling water at an altitude of 5000 feet, where the boiling point of water is 94.9 C?
min
Submit Answer
3 question attempts remaining
Autosaved at 9:31 AM
Nex
Back
9:31 AM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning