Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
need help with finding theoretical mass of aspirin and percent yield
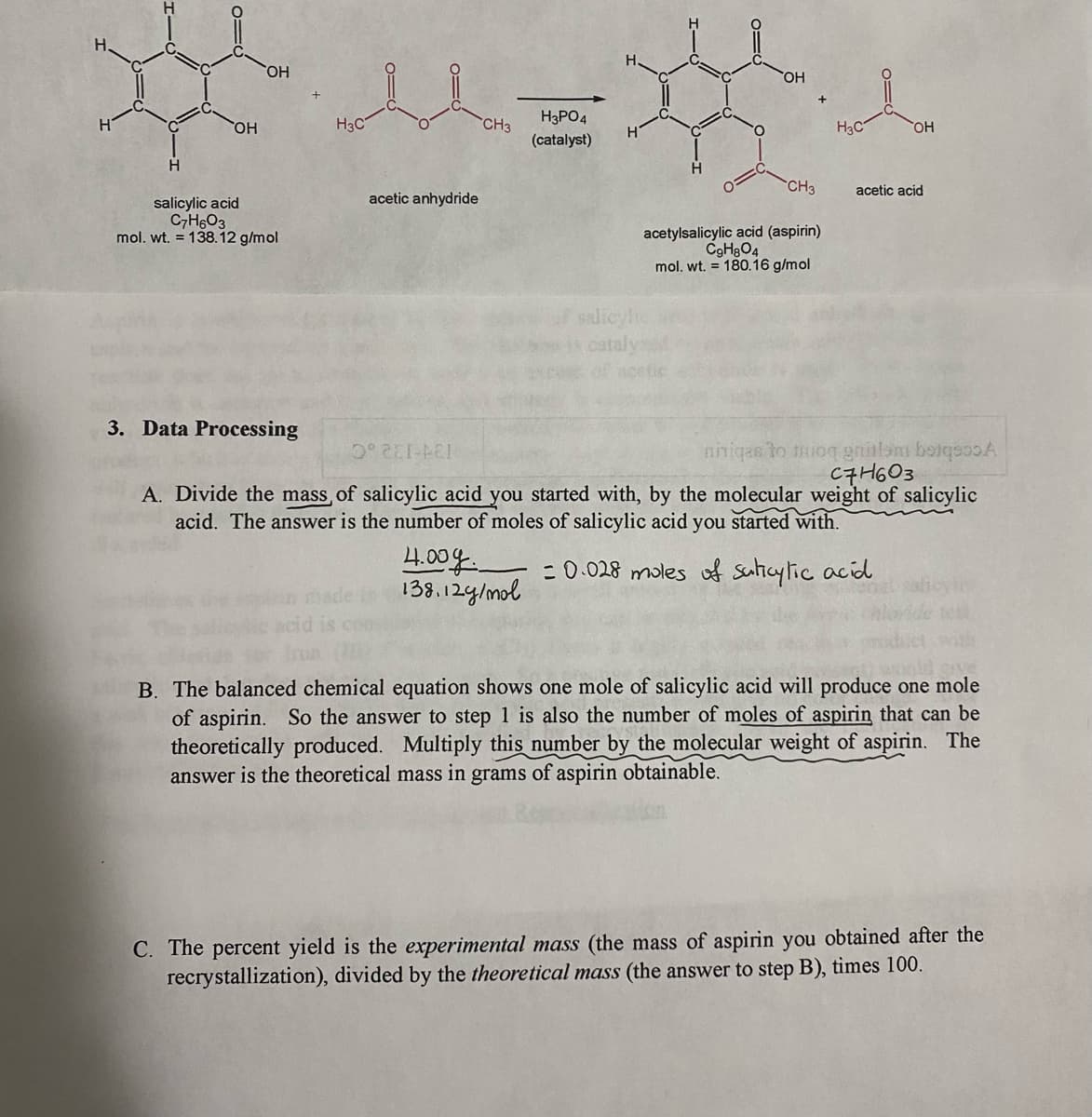
Transcribed Image Text:HO,
H3PO4
HO,
H3C
CH3
H3C
(catalyst)
CH3
acetic acid
acetic anhydride
salicylic acid
C7H6O3
mol. wt. = 138.12 g/mol
acetylsalicylic acid (aspirin)
C9H3O4
mol. wt. = 180.16 g/mol
salicylh
3. Data Pro
sing
nniqas to mioq gnialom botqsooA
C7H603
A. Divide the mass, of salicylic acid you started with, by the molecular weight of salicylic
134-132.C
acid. The answer is the number of moles of salicylic acid you started with.
4.00g
138.12g/mol
: 0.028 moles f sulicylic acid
coo
B. The balanced chemical equation shows one mole of salicylic acid will produce one mole
of aspirin. So the answer to step 1 is also the number of moles of aspirin that can be
theoretically produced. Multiply this number by the molecular weight of aspirin. The
answer is the theoretical mass in grams of aspirin obtainable.
C. The percent yield is the experimental mass (the mass of aspirin you obtained after the
recrystallization), divided by the theoretical mass (the answer to step B), times 100.
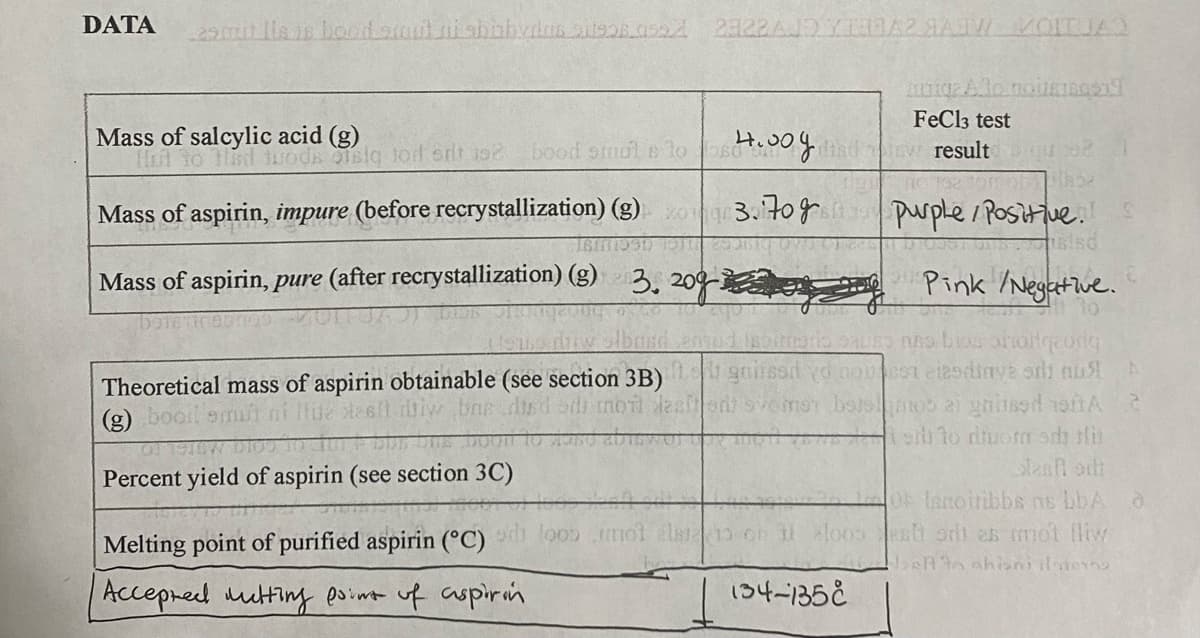
Transcribed Image Text:DATA
FeCl3 test
Mass of salcylic acid (g)
H to Hsd woda sig tod orit 102
4.00
bood smol e lo s
ew result
.
purple/ Positiue.S
sisd
Mass of aspirin, impure (before recrystallization) (g) 3.70g
Mass of aspirin, pure (after recrystallization) (g) 3. 209- e Pink /Negetive.
Theoretical mass of aspirin obtainable (see section 3B) gnsod yd noeSt aiodinve ori nu
(g) booit
toi to diuorm sch Hi
Percent yield of aspirin (see section 3C)
O lanoiribbs ns bbA
sh orh as mmol liw
Melting point of purified aspirin (°C)
Accepred mutting poimt uf aspirin
134-135E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

