NH2 HO Atenolol Tenormin® Below is a two-step reaction sequence. What are the steps in the synthesis? `NH2 -NH2 `NH2 base OH Он O The phenolate reacts with the epoxide preferentially over the alkyl halide at the less sterically hindered position. Then, the sterically hindered amine attacks the alkyl chloride. The phenolate reacts with the epoxide preferentially over the alkyl halide at the more sterically hindered position. Then, the sterically hindered amine attacks the alkyl chloride.
NH2 HO Atenolol Tenormin® Below is a two-step reaction sequence. What are the steps in the synthesis? `NH2 -NH2 `NH2 base OH Он O The phenolate reacts with the epoxide preferentially over the alkyl halide at the less sterically hindered position. Then, the sterically hindered amine attacks the alkyl chloride. The phenolate reacts with the epoxide preferentially over the alkyl halide at the more sterically hindered position. Then, the sterically hindered amine attacks the alkyl chloride.
Chapter23: Carbonyl Condensation Reactions
Section23.SE: Something Extra
Problem 45MP
Related questions
Question
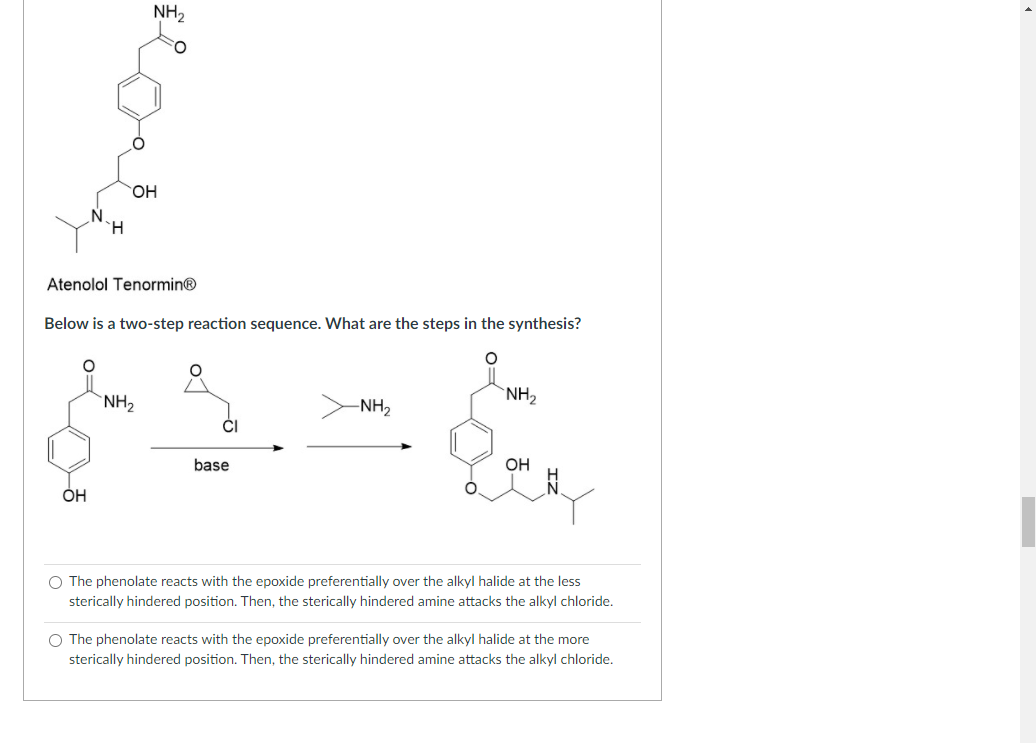
Transcribed Image Text:NH2
HO,
H.
Atenolol Tenormin®
Below is a two-step reaction sequence. What are the steps in the synthesis?
`NH2
-NH2
`NH2
base
OH
OH
O The phenolate reacts with the epoxide preferentially over the alkyl halide at the less
sterically hindered position. Then, the sterically hindered amine attacks the alkyl chloride.
O The phenolate reacts with the epoxide preferentially over the alkyl halide at the more
sterically hindered position. Then, the sterically hindered amine attacks the alkyl chloride.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning