Nitric oxide is an air pollutant found in car exhaust (as exhibited in the recent Volkswagen scandal). At the high temperatures found in car engines it is formed from the components of air: N2(g) + 02(g) = 2NO(g) (a) Using the data in Chang Appendix B, calculate the equilibrium constant for this reaction at 25°C and 1 atm. (b) 1500°C is a typical temperature inside a car engine after it has been running for some time. Calculate the equilibrium constant for the production of nitric oxide at this higher temperature. State any assumptions you need to make in order to complete the calculation. (Even though the temperature change is large here, assume that AHrxn, ASrxn can be treated as temperature independent.)
Nitric oxide is an air pollutant found in car exhaust (as exhibited in the recent Volkswagen scandal). At the high temperatures found in car engines it is formed from the components of air: N2(g) + 02(g) = 2NO(g) (a) Using the data in Chang Appendix B, calculate the equilibrium constant for this reaction at 25°C and 1 atm. (b) 1500°C is a typical temperature inside a car engine after it has been running for some time. Calculate the equilibrium constant for the production of nitric oxide at this higher temperature. State any assumptions you need to make in order to complete the calculation. (Even though the temperature change is large here, assume that AHrxn, ASrxn can be treated as temperature independent.)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.104QE: What is the sign of the standard Gibbs free-energy change at low temperatures and at high...
Related questions
Question
Transcribed Image Text:АPPENDI х В
+
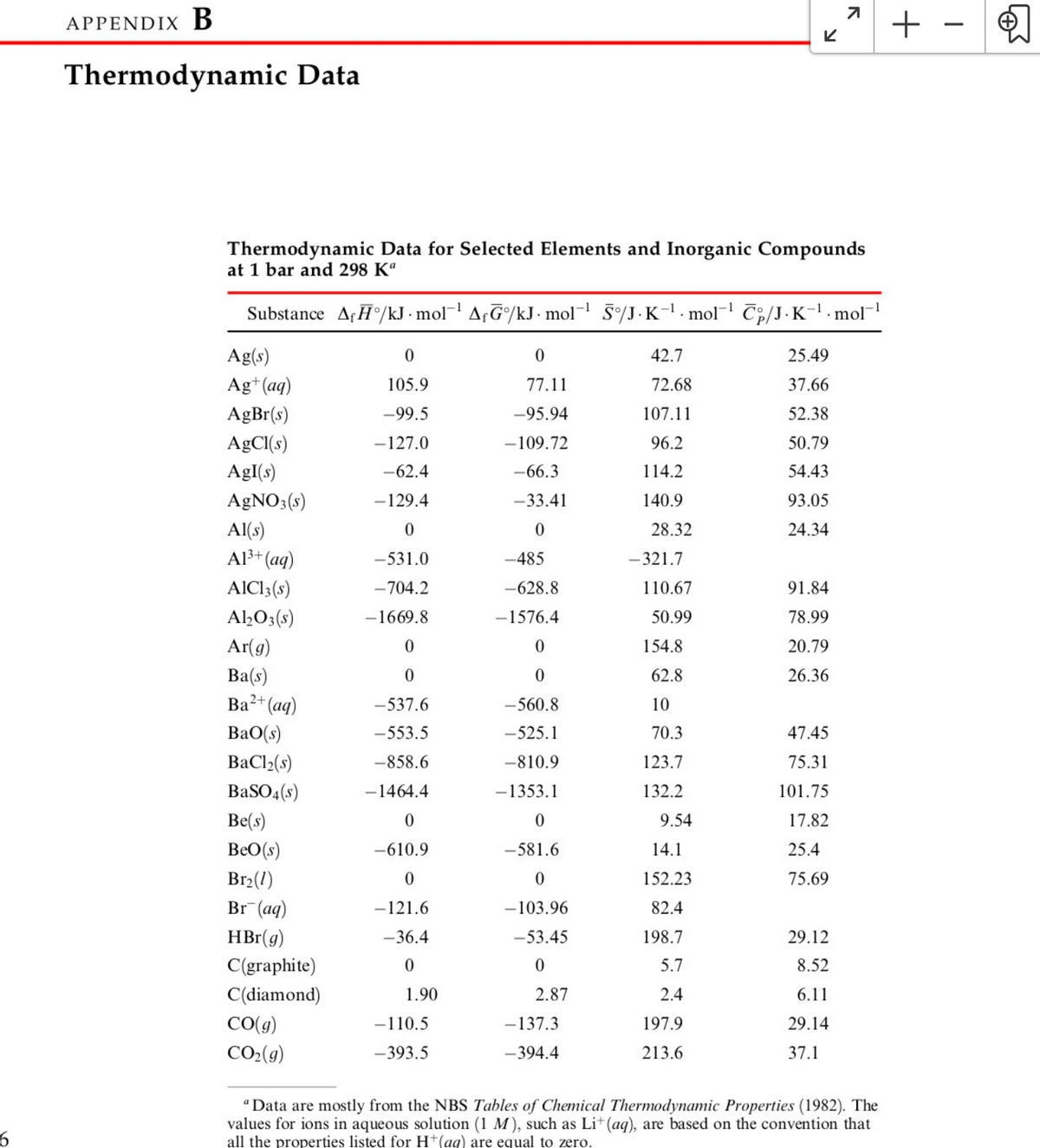
Thermodynamic Data
Thermodynamic Data for Selected Elements and Inorganic Compounds
at 1 bar and 298 K“
Substance AfĦ°/kJ · mol¯! Af&/kJ· mol¯ §/J·K-1. mol T;,/J·K-1 .mol-
Ag(s)
42.7
25.49
Ag+(aq)
105.9
77.11
72.68
37.66
AgBr(s)
-99.5
-95.94
107.11
52.38
AgCl(s)
-127.0
-109.72
96.2
50.79
AgI(s)
-62.4
-66.3
114.2
54.43
AgNO3(s)
-129.4
-33.41
140.9
93.05
Al(s)
28.32
24.34
Al3+ (aq)
-531.0
-485
-321.7
AICI3(s)
-704.2
-628.8
110.67
91.84
Al,O3(s)
-1669.8
-1576.4
50.99
78.99
Ar(g)
Ba(s)
Ba2+(aq)
154.8
20.79
62.8
26.36
-537.6
-560.8
10
BaO(s)
-553.5
-525.1
70.3
47.45
BaCl2(s)
-858.6
-810.9
123.7
75.31
BaSO (s)
-1464.4
-1353.1
132.2
101.75
Be(s)
9.54
17.82
BeO(s)
-610.9
-581.6
14.1
25.4
Br2(1)
152.23
75.69
Br (aq)
-121.6
-103.96
82.4
HBr(g)
-36.4
-53.45
198.7
29.12
C(graphite)
5.7
8.52
C(diamond)
1.90
2.87
2.4
6.11
CO(g)
-110.5
-137.3
197.9
29.14
CO2(9)
-393.5
-394.4
213.6
37.1
"Data are mostly from the NBS Tables of Chemical Thermodynamic Properties (1982). The
values for ions in aqueous solution (1 M), such as Li+(aq), are based on the convention that
all the properties listed for H+(ag) are equal to zero.

Transcribed Image Text:Nitric oxide is an air pollutant found in car exhaust (as exhibited in the recent Volkswagen
scandal). At the high temperatures found in car engines it is formed from the components of air:
N2(g) + 02(g) = 2NO(g)
(a) Using the data in Chang Appendix B, calculate the equilibrium constant for this reaction at
25°C and 1 atm.
(b) 1500°C is a typical temperature inside a car engine after it has been running for some time.
Calculate the equilibrium constant for the production of nitric oxide at this higher temperature.
State any assumptions you need to make in order to complete the calculation. (Even though the
temperature change is large here, assume that AHrxn, ASrxn can be treated as temperature
independent.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning