Carbon dioxide is liberated by the reaction of aqueous hydrochloric acid with calcium carbonate: CaCO3(s) + 2 H* (aq) → Ca²+ (aq) + CO2(g) + H,O(e) A volume of 722 mL CO2(g) is collected over water at 20°C and a total pressure of 0.9963 atm. At this tempera- ture, water has a vapor pressure of 0.0231 atm. Calculate the mass of calcium carbonate that has reacted, assuming no losses of carbon dioxide.
Carbon dioxide is liberated by the reaction of aqueous hydrochloric acid with calcium carbonate: CaCO3(s) + 2 H* (aq) → Ca²+ (aq) + CO2(g) + H,O(e) A volume of 722 mL CO2(g) is collected over water at 20°C and a total pressure of 0.9963 atm. At this tempera- ture, water has a vapor pressure of 0.0231 atm. Calculate the mass of calcium carbonate that has reacted, assuming no losses of carbon dioxide.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.114P: 5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous...
Related questions
Question
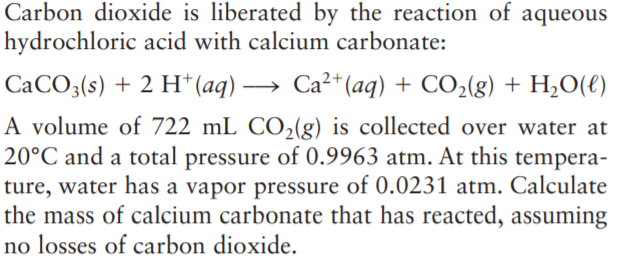
Transcribed Image Text:Carbon dioxide is liberated by the reaction of aqueous
hydrochloric acid with calcium carbonate:
CaCO3(s) + 2 H* (aq) → Ca²+ (aq) + CO2(g) + H,O(e)
A volume of 722 mL CO2(g) is collected over water at
20°C and a total pressure of 0.9963 atm. At this tempera-
ture, water has a vapor pressure of 0.0231 atm. Calculate
the mass of calcium carbonate that has reacted, assuming
no losses of carbon dioxide.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 7 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning