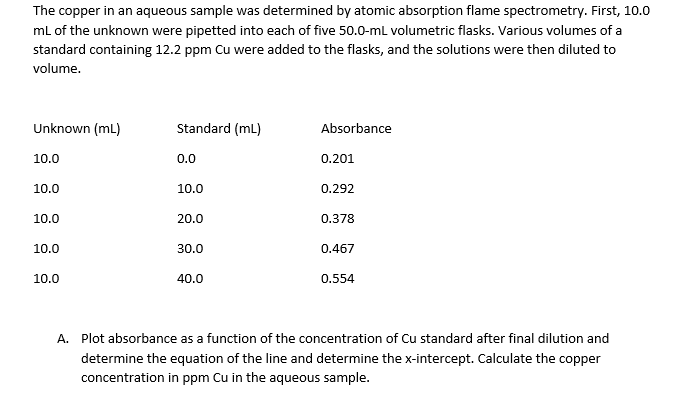
nL of the unknown were pipetted into each of five 50.0-ml volumetric flasks. Various volumes of a tandard containing 12.2 ppm Cu were added to the flasks, and the solutions were then diluted to olume. Unknown (mL) L0.0 Standard (mL) Absorbance 0.0 0.201 L0.0 10.0 0.292 0.0 20.0 0.378 0.0 30.0 0.467 L0.0 40.0 0.554 A. Plot absorbance as a function of the concentration of Cu standard after final dilution and determine the equation of the line and determine the x-intercept. Calculate the copper concentration in ppm Cu in the aqueous sample.
nL of the unknown were pipetted into each of five 50.0-ml volumetric flasks. Various volumes of a tandard containing 12.2 ppm Cu were added to the flasks, and the solutions were then diluted to olume. Unknown (mL) L0.0 Standard (mL) Absorbance 0.0 0.201 L0.0 10.0 0.292 0.0 20.0 0.378 0.0 30.0 0.467 L0.0 40.0 0.554 A. Plot absorbance as a function of the concentration of Cu standard after final dilution and determine the equation of the line and determine the x-intercept. Calculate the copper concentration in ppm Cu in the aqueous sample.
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.15QAP
Related questions
Question
Please answer in detailed solution.
Transcribed Image Text:The copper in an aqueous sample was determined by atomic absorption flame spectrometry. First, 10.0
ml of the unknown were pipetted into each of five 50.0-ml volumetric flasks. Various volumes of a
standard containing 12.2 ppm Cu were added to the flasks, and the solutions were then diluted to
volume.
Unknown (mL)
Standard (mL)
Absorbance
10.0
0.0
0.201
10.0
10.0
0.292
10.0
20.0
0.378
10.0
30.0
0.467
10.0
40.0
0.554
A. Plot absorbance as a function of the concentration of Cu standard after final dilution and
determine the equation of the line and determine the x-intercept. Calculate the copper
concentration in ppm Cu in the aqueous sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
