One of the inherently satisfying features of chemistry is that chemical materials form and interact in a rational and predictable manner. For example, one can predict a great deal about a particular kind of molecule by experimentally determining the percentage composition of the elements in that compound. This gives us the relative proportions of the elements in the molecule. For a molecule made up of elements A, B, and C, A compound is 40.0% C, 6.70% H, and 53.3% O by mass. Assume that we have a 100.-g sample of this compound. Part A the proportions might be A:B:C2, meaning that there are two atoms of C for each atom of A and each atom of B. This may not be the actual description of the molecule (which might actually be A2B2 C4), but it is the "reduced" version of that formula, called the empirical formula. The actual formula is some multiple of the empirical formula. To know the actual formula we need to know both What are the subscripts in the empirical formula of this compound? Enter the subscripts for C, H, and O, respectively, separated by commas (e.g., 5,6,7). • View Available Hint(s) the empirical formula and the molecular mass of the compound. This provides us with the multiplier value in whole units that must be applied to the empirical formula to get the actual formula.
One of the inherently satisfying features of chemistry is that chemical materials form and interact in a rational and predictable manner. For example, one can predict a great deal about a particular kind of molecule by experimentally determining the percentage composition of the elements in that compound. This gives us the relative proportions of the elements in the molecule. For a molecule made up of elements A, B, and C, A compound is 40.0% C, 6.70% H, and 53.3% O by mass. Assume that we have a 100.-g sample of this compound. Part A the proportions might be A:B:C2, meaning that there are two atoms of C for each atom of A and each atom of B. This may not be the actual description of the molecule (which might actually be A2B2 C4), but it is the "reduced" version of that formula, called the empirical formula. The actual formula is some multiple of the empirical formula. To know the actual formula we need to know both What are the subscripts in the empirical formula of this compound? Enter the subscripts for C, H, and O, respectively, separated by commas (e.g., 5,6,7). • View Available Hint(s) the empirical formula and the molecular mass of the compound. This provides us with the multiplier value in whole units that must be applied to the empirical formula to get the actual formula.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question
100%
Please answer question 17 Part A
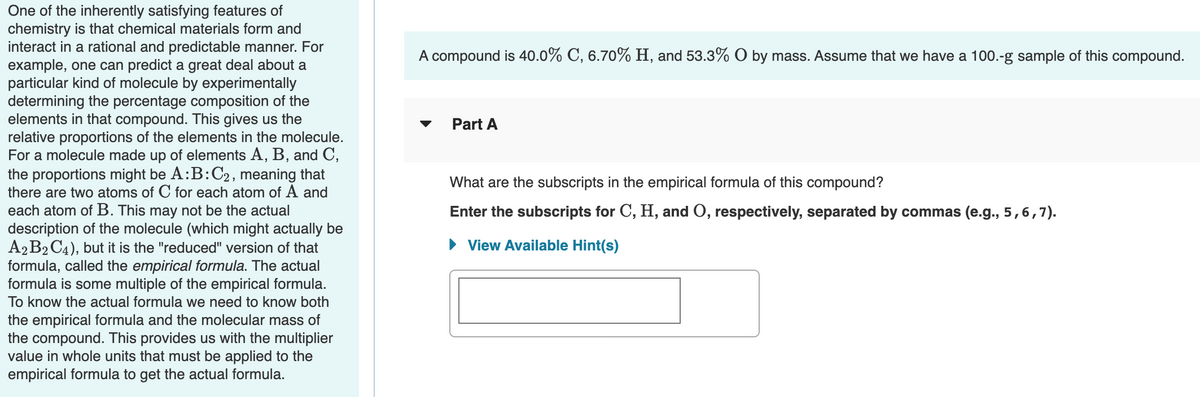
Transcribed Image Text:One of the inherently satisfying features of
chemistry is that chemical materials form and
interact in a rational and predictable manner. For
example, one can predict a great deal about a
particular kind of molecule by experimentally
determining the percentage composition of the
elements in that compound. This gives us the
relative proportions of the elements in the molecule.
For a molecule made up of elements A, B, and C,
the proportions might be A:B:C2, meaning that
there are two atoms of C for each atom of A and
A compound is 40.0% C, 6.70% H, and 53.3% O by mass. Assume that we have a 100.-g sample of this compound.
Part A
What are the subscripts in the empirical formula of this compound?
each atom of B. This may not be the actual
description of the molecule (which might actually be
A2B2 C4), but it is the "reduced" version of that
formula, called the empirical formula. The actual
formula is some multiple of the empirical formula.
Enter the subscripts for C, H, and O, respectively, separated by commas (e.g., 5,6,7).
• View Available Hint(s)
To know the actual formula we need to know both
the empirical formula and the molecular mass of
the compound. This provides us with the multiplier
value in whole units that must be applied to the
empirical formula to get the actual formula.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax