Now let's consider histidine as a free amino acid in aqueous solution. Draw the most likely structure of histidine under biochemical standard state conditions. Given that free histidine has the following three pK, values, assign each to its corresponding acidic hydrogen or conjugate base in your structure from part a). pK1 = 1.7, pK22 = 6.0, pK33 = 9.1 For each pKa, give the corresponding expression for the equilibrium constant.
Now let's consider histidine as a free amino acid in aqueous solution. Draw the most likely structure of histidine under biochemical standard state conditions. Given that free histidine has the following three pK, values, assign each to its corresponding acidic hydrogen or conjugate base in your structure from part a). pK1 = 1.7, pK22 = 6.0, pK33 = 9.1 For each pKa, give the corresponding expression for the equilibrium constant.
Chapter26: Biomolecules: Amino Acids, Peptides, And Proteins
Section26.SE: Something Extra
Problem 57AP: Oxytocin, a nonapeptide hormone secreted by the pituitary gland, functions by stimulating uterine...
Related questions
Question
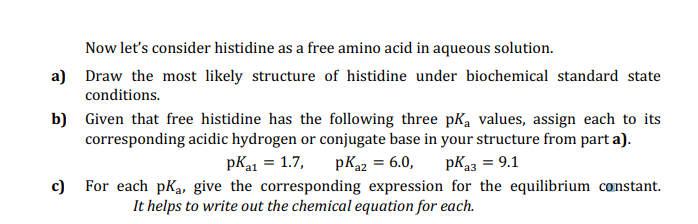
Transcribed Image Text:Now let's consider histidine as a free amino acid in aqueous solution.
a) Draw the most likely structure of histidine under biochemical standard state
conditions.
b) Given that free histidine has the following three pKa values, assign each to its
corresponding acidic hydrogen or conjugate base in your structure from part a).
pK1 = 1.7,
pKa2 = 6.0,
pK23 = 9.1
c)
For each pKa, give the corresponding expression for the equilibrium constant.
It helps to write out the chemical equation for each.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

