Based on the given figure, a. what is the possible identity of the amino acid? b. what is the isoelectric point of AA c. what is the pKa corresponding to the dissociation of alpha carboxylic group
Based on the given figure, a. what is the possible identity of the amino acid? b. what is the isoelectric point of AA c. what is the pKa corresponding to the dissociation of alpha carboxylic group
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
Based on the given figure,
a. what is the possible identity of the amino acid?
b. what is the isoelectric point of AA
c. what is the pKa corresponding to the dissociation of alpha carboxylic group
d. where is the region or point where AA is predominantly present as a (-2) charged species
e. where is the effective buffering range for the amino acid on the acidic region
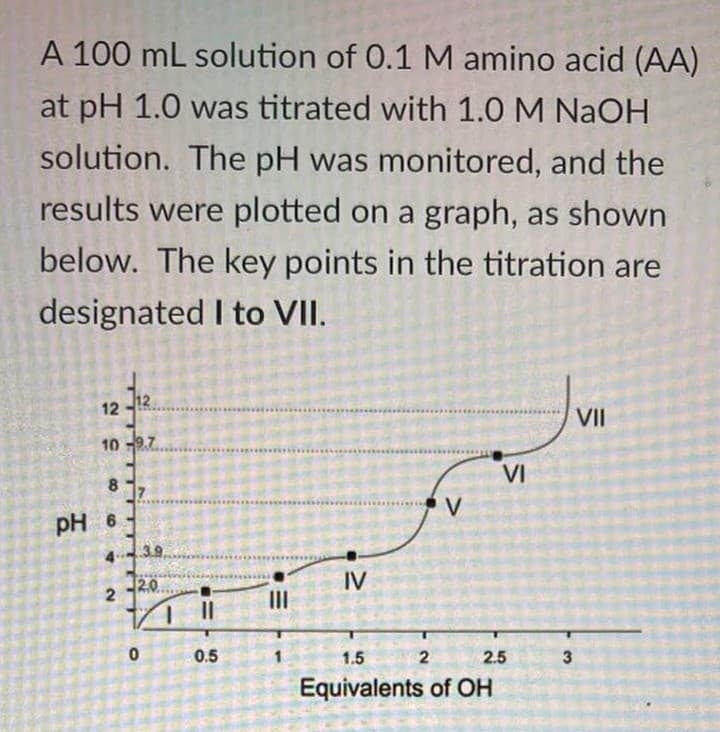
Transcribed Image Text:A 100 mL solution of 0.1 M amino acid (AA)
at pH 1.0 was titrated with 1.0 M NaOH
solution. The pH was monitored, and the
results were plotted on a graph, as shown
below. The key points in the titration are
designated I to VII.
12
VII
10 97
VI
8.
V
pH 6
43.9
20
IV
II
0.5
1.5
2
2.5
Equivalents of OH
3.
2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning