Part B Complete the sentences to explain your answers. Reset Help particles According to Rutherford's nuclear theory, the core of an atom (nucleus) contains most of the mass positively charged of an atom and is positively charged so the majority of the mass of a fluorine atom cannot be due to its nine electrons. empty space more than According to Rutherford's nuclear theory, most of the volume of an atom is empty space so the volume of a hydrogen atom cannot be mostly due to the proton. negatively charged the same as According to Rutherford's nuclear theory, the number of negatively charged particles outside the nucleus is the same as the number of positively charged particles within the nucleus, so a nitrogen mass atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150 dense electrons. less than Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining You filled in 2 of 4 blanks incorrectly. If the core of an atom had contained most of the particles of an atom, that would have resembled Thomson's model. Ru negatively charged particles in an atom was the same; however, neutrons were not accounted for and, thus, some of the particles in an atom were unaccoun First launch this video. During the video, you'll be asked a conceptual question about the example. After watching the video, try the question again. You can w
Part B Complete the sentences to explain your answers. Reset Help particles According to Rutherford's nuclear theory, the core of an atom (nucleus) contains most of the mass positively charged of an atom and is positively charged so the majority of the mass of a fluorine atom cannot be due to its nine electrons. empty space more than According to Rutherford's nuclear theory, most of the volume of an atom is empty space so the volume of a hydrogen atom cannot be mostly due to the proton. negatively charged the same as According to Rutherford's nuclear theory, the number of negatively charged particles outside the nucleus is the same as the number of positively charged particles within the nucleus, so a nitrogen mass atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150 dense electrons. less than Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining You filled in 2 of 4 blanks incorrectly. If the core of an atom had contained most of the particles of an atom, that would have resembled Thomson's model. Ru negatively charged particles in an atom was the same; however, neutrons were not accounted for and, thus, some of the particles in an atom were unaccoun First launch this video. During the video, you'll be asked a conceptual question about the example. After watching the video, try the question again. You can w
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 102AP
Related questions
Question
100%
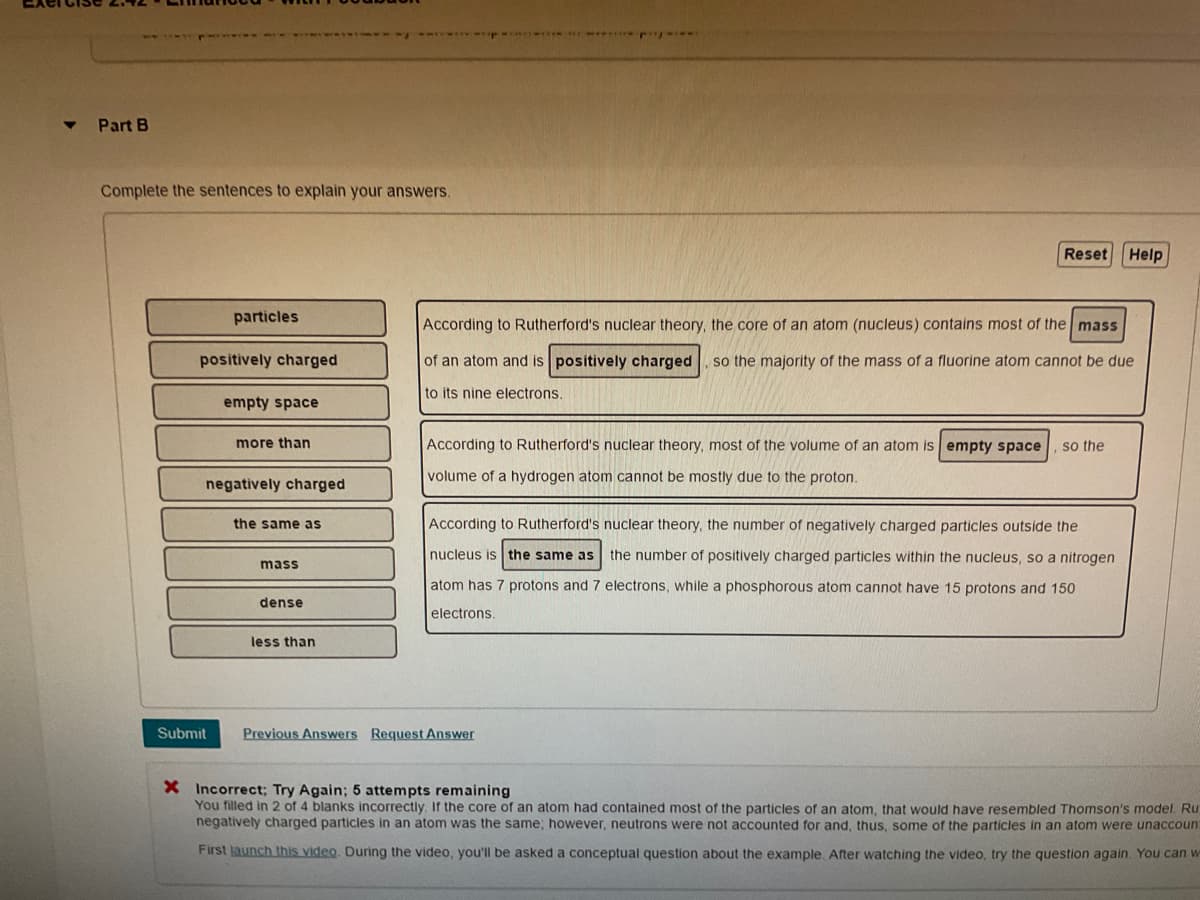
Transcribed Image Text:Part B
Complete the sentences to explain your answers.
Reset Help
particles
According to Rutherford's nuclear theory, the core of an atom (nucleus) contains most of the mass
positively charged
of an atom and is positively charged
so the majority of the mass of a fluorine atom cannot be due
to its nine electrons.
empty space
more than
According to Rutherford's nuclear theory, most of the volume of an atom is empty space
so the
volume of a hydrogen atom cannot be mostly due to the proton.
negatively charged
the same as
According to Rutherford's nuclear theory, the number of negatively charged particles outside the
nucleus is the same as the number of positively charged particles within the nucleus, so a nitrogen
mass
atom has 7 protons and 7 electrons, while a phosphorous atom cannot have 15 protons and 150
dense
electrons.
less than
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
You filled in 2 of 4 blanks incorrectly. If the core of an atom had contained most of the particles of an atom, that would have resembled Thomson's model. Ru
negatively charged particles in an atom was the same; however, neutrons were not accounted for and, thus, some of the particles in an atom were unaccount
First launch this video. During the video, you'll be asked a conceptual question about the example, After watching the video, try the question again. You can w
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning