Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 81QAP
Related questions
Question
Number 2
Transcribed Image Text:Is
Table
Window
Help
AutoSave
OFF
Document3
Insert
Draw
Design
Layout
References
Mailings
Review
>> Q Tell me
E Share
Comme
Calibri (Bo...
11
A A
Aa v
B
U
v ab x, x
A
A v
三。
Styles Styles
Pane
Dictate
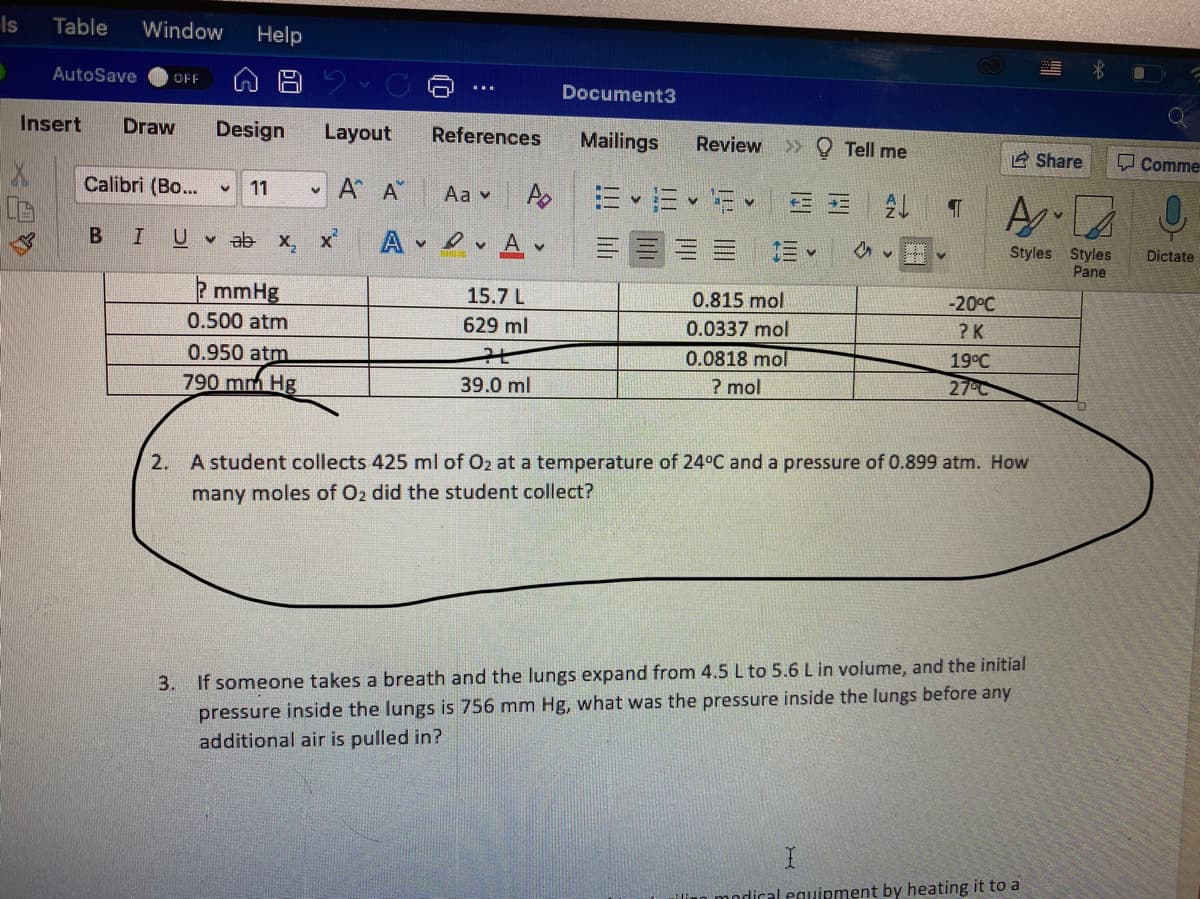
2mmHg
15.7 L
0.815 mol
-20°C
0.500 atm
629 ml
0.0337 mol
? K
0.950 atm
0.0818 mol
19°C
790 mm Hg
39.0 ml
? mol
27°C
2. A student collects 425 ml of Oz at a temperature of 24°C and a pressure of 0.899 atm. How
many moles of Oz did the student collect?
3. If someone takes a breath and the lungs expand from 4.5 L to 5.6 L in volume, and the initial
pressure inside the lungs is 756 mm Hg, what was the pressure inside the lungs before any
additional air is pulled in?
modical eguipment by heating it to a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax