• Use the diagram below to answer the questions on the following page: o What is its atomic mass? Nucleus 3 o Assuming that the atom on the previous page is electrically neutral (which it is), what is the atomic number of this atom (as shown on bottom of page 2)? o What atom is this? Electron o Is this atom chemically active or inert? How do you know? o How many electrons are needed to fill its outer (valence) shell?
• Use the diagram below to answer the questions on the following page: o What is its atomic mass? Nucleus 3 o Assuming that the atom on the previous page is electrically neutral (which it is), what is the atomic number of this atom (as shown on bottom of page 2)? o What atom is this? Electron o Is this atom chemically active or inert? How do you know? o How many electrons are needed to fill its outer (valence) shell?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 2ALQ: rite the proper charges so that an alkali metal, a noble gas, and a halogen have the same electron...
Related questions
Question
please answer with a bit of explanation for me to know how did you arrive to that answer. thank you
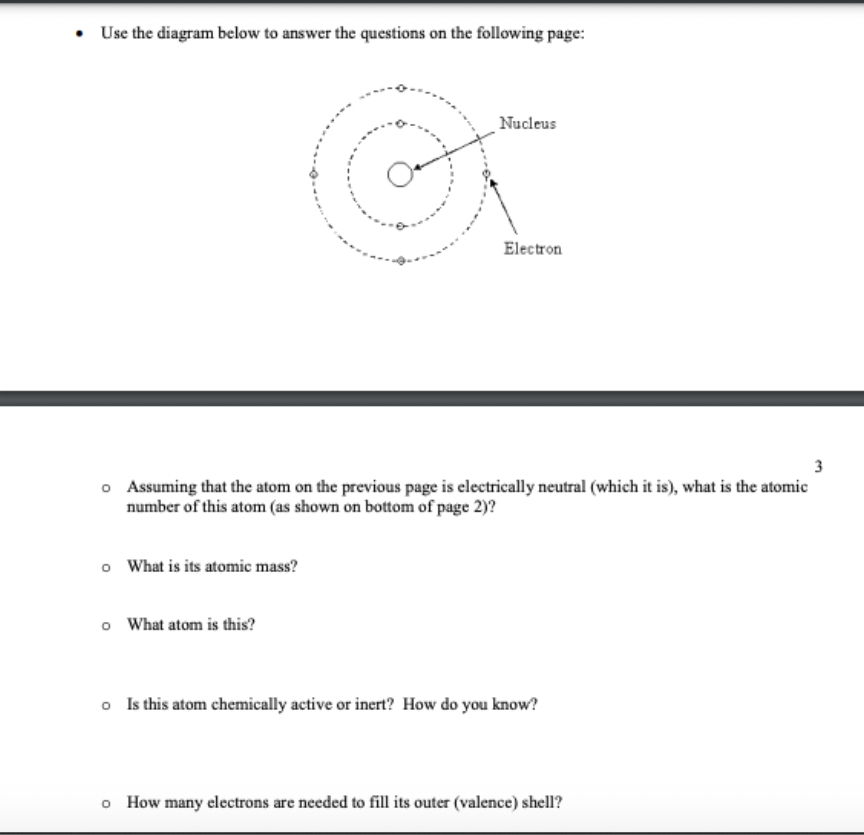
Transcribed Image Text:• Use the diagram below to answer the questions on the following page:
Nucleus
Electron
3
o Assuming that the atom on the previous page is electrically neutral (which it is), what is the atomic
number of this atom (as shown on bottom of page 2)?
o What is its atomic mass?
o What atom is this?
o Is this atom chemically active or inert? How do you know?
o How many electrons are needed to fill its outer (valence) shell?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning