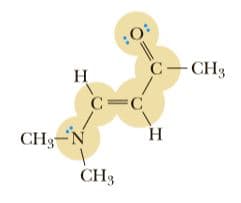
What is the hybridization of the highlighted atoms in the following structure? What are your estimates for the bond angles around these highlighted atoms? in what kind of orbital does the lone pair of electrons on the nitrogen reside?
Mixing of atomic orbital to the new hybrid orbital is known as hybridization.
If a C atom contains a C=C double bond, then it is sp2-hybridized
If a C atom contains a C-C single bond, then it is sp3-hybridized
If a C atom contains a 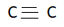 triple bond, then it is sp-hybridized
triple bond, then it is sp-hybridized
The given structure is shown below:
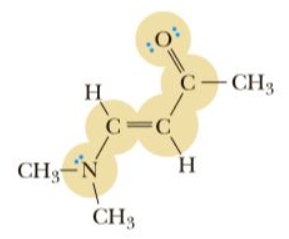
The hybridization of highlighted atoms are shown below:
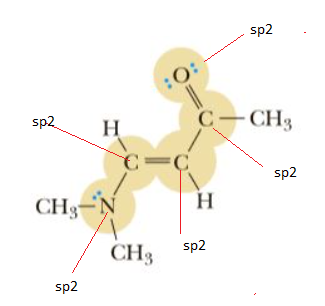
If a C atom contains a C=C double bond, then it is sp2-hybridized
If a C atom contains a C=O double bond, then it is sp2-hybridized
N atom is sp2 hybridized as it contains one lone pair.
Step by step
Solved in 5 steps with 4 images









