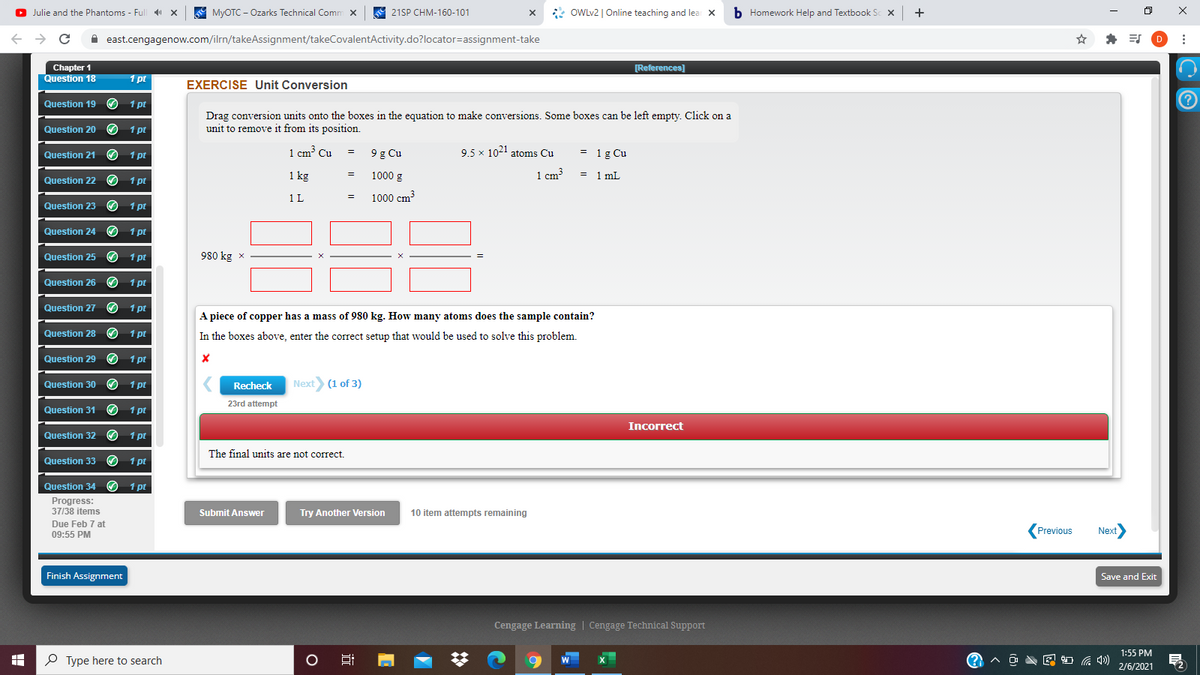
O Julie and the Phantoms - Full 4 x A MYOTC - Ozarks Technical Comm x S 21SP CHM-160-101 * OWLV2 | Online teaching and lea x b Homework Help and Textbook + i east.cengagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take D Chapter [References) Question 18 1 pt EXERCISE Unit Conversion Question 19 O 1 pt Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. Question 20 1 pt 1 cm Cu = 9g Cu 9.5 x 1021 atoms Cu = 1g Cu Question 21 の 1pt u 1 kg 1000 g 1 cm³ = 1 mL Question 22 1 pt 1L 1000 cm Question 23 O 1 pt Question 24 O 1 pt Question 25 O 1 pt 980 kg x Question 26 O 1 pt Question 27 1 pt A piece of copper has a mass of 980 kg. How many atoms does the sample contain? In the boxes above, enter the correct setup that would be used to solve this problem. Question 28 O 1 pt Question 29 O t Question 30 O 1 pt Recheck Next) (1 of 3) 23rd attempt Question 31 O 1 pt Incorrect Question 32 O 1 pt The final units are not correct. Question 33 の 1 pt Question 34 O 1 pr Progress: 37/38 items Submit Answer Try Another Version 10 item attempts remaining Due Feb 7 at 09:55 PM (Previous Next Finish Assignment Save and Exit Cengage Learning | Cengage Technical Support 1:55 PM P Type here to search O Ei 日 2/6/2021
Reactions of Ethers
Ethers (R-O-R’) are compounds formed by replacing hydrogen atoms of an alcohol (R-OH compound) or a phenol (C6H5OH) by an aryl/ acyl group (functional group after removing single hydrogen from an aromatic ring). In this section, reaction, preparation and behavior of ethers are discussed in the context of organic chemistry.
Epoxides
Epoxides are a special class of cyclic ethers which are an important functional group in organic chemistry and generate reactive centers due to their unusual high reactivity. Due to their high reactivity, epoxides are considered to be toxic and mutagenic.
Williamson Ether Synthesis
An organic reaction in which an organohalide and a deprotonated alcohol forms ether is known as Williamson ether synthesis. Alexander Williamson developed the Williamson ether synthesis in 1850. The formation of ether in this synthesis is an SN2 reaction.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images


