O KINETICS AND EQUILIBRIUM Predicting relative forward and reverse rates of reaction in a dyn... Acetic acid and water react to form hydronium cation and acetate anion, like this: HCH3CO2(aq)+H2O)H3 (a)+CH,CO2 (aq) Imagine 182. mmol of CH3CO2 are removed from a flask containing a mixture of HCH3CO, H20, H0 and CH3CO2 at equilibrium, and then answer he following questions Zero Greater than zero, but less than the rate of the forward reaction Greater than zero, and equal to the rate of the forward reaction Greater than zero, and greater than the rate of the forward reaction Zero Greater than zero, but less than the rate of the forward reaction Greater than zero, and equal to the rate of the forward reaction Greater than zero, and greater than the rate of the forward reaction Zero Greater than zero, but less than the rate of the forward reaction Greater than zero, and equal to the rate of the forward reaction Greater than zero, and greater than the rate of the forward reaction None Some, but less than 182. mmol 182. mmol More than 182. mmol What is the rate of the reverse reaction before any CH3CO2 has been removed from the flask? What is the rate of the reverse reaction just after the CH3CO2 has been removed from the flask? What is the rate of the reverse reaction when the system has again reached equilibrium? How much less CH3CO2 is in the flask when the system has again reached equilibrium?
O KINETICS AND EQUILIBRIUM Predicting relative forward and reverse rates of reaction in a dyn... Acetic acid and water react to form hydronium cation and acetate anion, like this: HCH3CO2(aq)+H2O)H3 (a)+CH,CO2 (aq) Imagine 182. mmol of CH3CO2 are removed from a flask containing a mixture of HCH3CO, H20, H0 and CH3CO2 at equilibrium, and then answer he following questions Zero Greater than zero, but less than the rate of the forward reaction Greater than zero, and equal to the rate of the forward reaction Greater than zero, and greater than the rate of the forward reaction Zero Greater than zero, but less than the rate of the forward reaction Greater than zero, and equal to the rate of the forward reaction Greater than zero, and greater than the rate of the forward reaction Zero Greater than zero, but less than the rate of the forward reaction Greater than zero, and equal to the rate of the forward reaction Greater than zero, and greater than the rate of the forward reaction None Some, but less than 182. mmol 182. mmol More than 182. mmol What is the rate of the reverse reaction before any CH3CO2 has been removed from the flask? What is the rate of the reverse reaction just after the CH3CO2 has been removed from the flask? What is the rate of the reverse reaction when the system has again reached equilibrium? How much less CH3CO2 is in the flask when the system has again reached equilibrium?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.58PAE
Related questions
Question
Kinetics & Equilibrium: Predicting relative forward and reverse
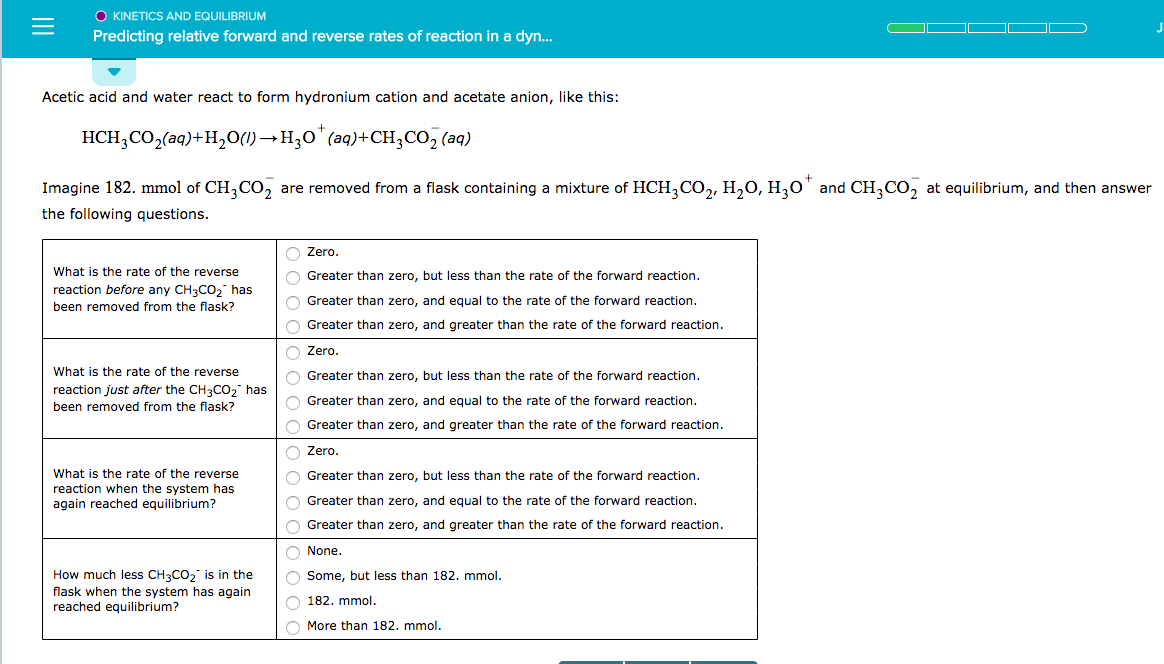
Transcribed Image Text:O KINETICS AND EQUILIBRIUM
Predicting relative forward and reverse rates of reaction in a dyn...
Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH3CO2(aq)+H2O)H3 (a)+CH,CO2 (aq)
Imagine 182. mmol of CH3CO2 are removed from a flask containing a mixture of HCH3CO, H20, H0 and CH3CO2 at equilibrium, and then answer
he following questions
Zero
Greater than zero, but less than the rate of the forward reaction
Greater than zero, and equal to the rate of the forward reaction
Greater than zero, and greater than the rate of the forward reaction
Zero
Greater than zero, but less than the rate of the forward reaction
Greater than zero, and equal to the rate of the forward reaction
Greater than zero, and greater than the rate of the forward reaction
Zero
Greater than zero, but less than the rate of the forward reaction
Greater than zero, and equal to the rate of the forward reaction
Greater than zero, and greater than the rate of the forward reaction
None
Some, but less than 182. mmol
182. mmol
More than 182. mmol
What is the rate of the reverse
reaction before any CH3CO2 has
been removed from the flask?
What is the rate of the reverse
reaction just after the CH3CO2 has
been removed from the flask?
What is the rate of the reverse
reaction when the system has
again reached equilibrium?
How much less CH3CO2 is in the
flask when the system has again
reached equilibrium?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning