O KINETICS AND EQUILIBRIUM Solving problems that mix equilibrium ideas with gas laws Nitrogen forms a surprising number of compounds with oxygen. A number of these, often given the collective symbol NOx (for "nitrogen + x oxygens") are serious contributors to air pollution. They can often be interconverted, sometimes by reaction with oxygen or ozone (02) in the air An atmospheric scientist decides to study the reaction between nitrogen dioxide and oxygen that produces dinitrogen pentoxide. She fills a stainless steel reaction chamber with 9.1 atm of nitrogen dioxide gas and 9.3 atm of oxygen gas and raises the temperature considerably. At equilibrium she measures the mole fraction of dinitrogen pentoxide to be 0.23. Calculate the pressure equilibrium constant K for the equilibrium between nitrogen dioxide, Oxygen, and dinitrogen pentoxide at the final temperature of the р mixture. Round your answer to 2 significant digits. x10 р ? Check Privac Explanation 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use X
O KINETICS AND EQUILIBRIUM Solving problems that mix equilibrium ideas with gas laws Nitrogen forms a surprising number of compounds with oxygen. A number of these, often given the collective symbol NOx (for "nitrogen + x oxygens") are serious contributors to air pollution. They can often be interconverted, sometimes by reaction with oxygen or ozone (02) in the air An atmospheric scientist decides to study the reaction between nitrogen dioxide and oxygen that produces dinitrogen pentoxide. She fills a stainless steel reaction chamber with 9.1 atm of nitrogen dioxide gas and 9.3 atm of oxygen gas and raises the temperature considerably. At equilibrium she measures the mole fraction of dinitrogen pentoxide to be 0.23. Calculate the pressure equilibrium constant K for the equilibrium between nitrogen dioxide, Oxygen, and dinitrogen pentoxide at the final temperature of the р mixture. Round your answer to 2 significant digits. x10 р ? Check Privac Explanation 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use X
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 94E: Xenon and fluorine will react to form binary compounds when a mixture of these two gases is heated...
Related questions
Question
Check out the pressure equilibrium constant KP for the equilibrium between nitrogen dioxide. Oxygen. And dinitrogen pentoxide has the final temperature of the mixture round your answer to two sig figs. Please check work
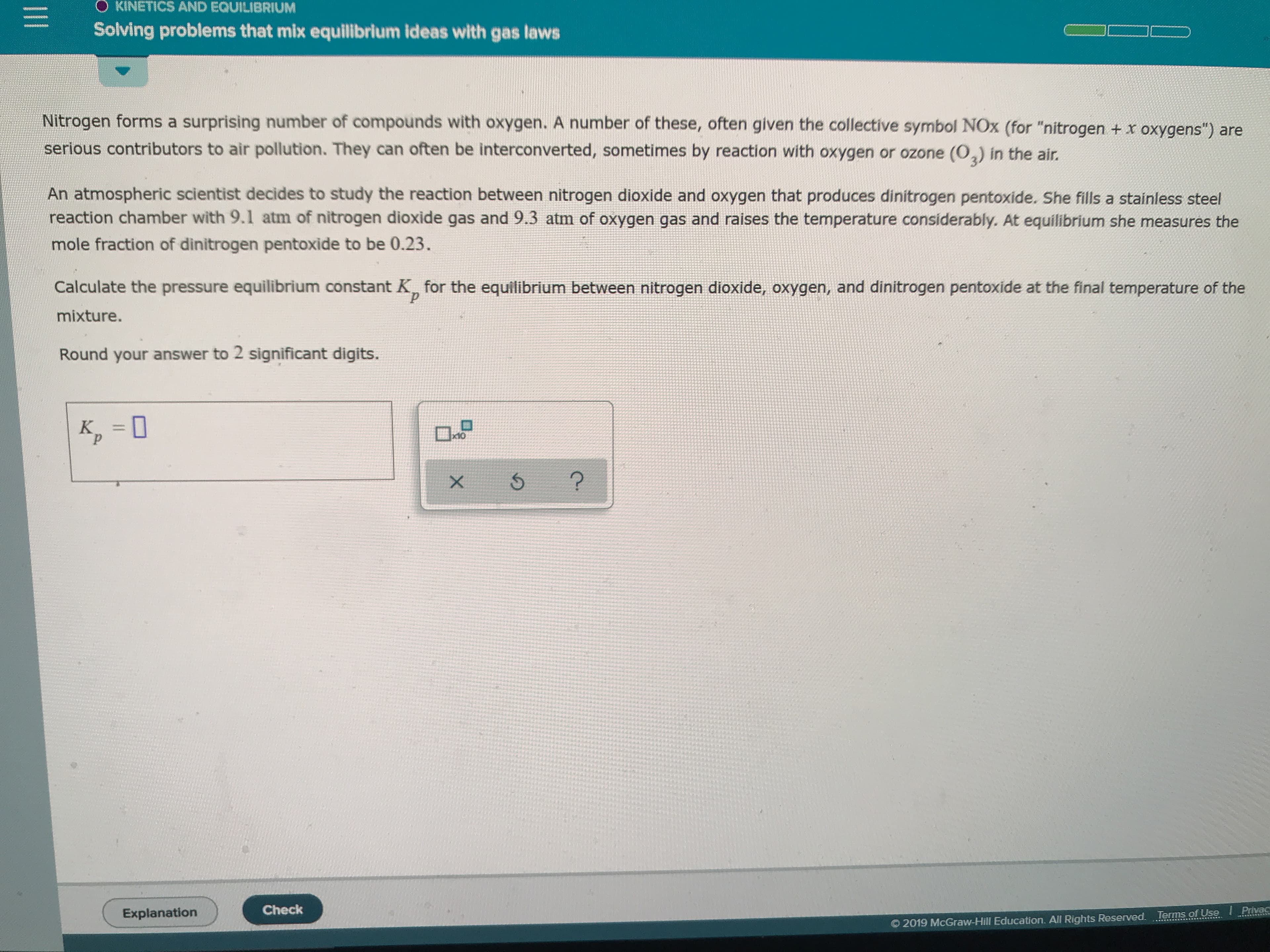
Transcribed Image Text:O KINETICS AND EQUILIBRIUM
Solving problems that mix equilibrium ideas with gas laws
Nitrogen forms a surprising number of compounds with oxygen. A number of these, often given the collective symbol NOx (for "nitrogen + x oxygens") are
serious contributors to air pollution. They can often be interconverted, sometimes by reaction with oxygen or ozone (02) in the air
An atmospheric scientist decides to study the reaction between nitrogen dioxide and oxygen that produces dinitrogen pentoxide. She fills a stainless steel
reaction chamber with 9.1 atm of nitrogen dioxide gas and 9.3 atm of oxygen gas and raises the temperature considerably. At equilibrium she measures the
mole fraction of dinitrogen pentoxide to be 0.23.
Calculate the pressure equilibrium constant K for the equilibrium between nitrogen dioxide, Oxygen, and dinitrogen pentoxide at the final temperature of the
р
mixture.
Round your answer to 2 significant digits.
x10
р
?
Check
Privac
Explanation
2019 McGraw-Hill Education. All Rights Reserved. Terms of Use
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning