Reactions of Alkyl and Aryl halides
In organic chemistry, an alkyl halide is formed when an atom of hydrogen is switched by a halogen in a hydrocarbon or aliphatic compound. An aryl halide is formed when an atom of hydrogen is substituted by a halogen atom in an aromatic compound. Metals react with aryl halides and alkyl halides and they also go through nucleophilic substitution reactions and elimination reactions.
Zaitsev's Rule in Organic Chemistry
Alexander Zaitsev (also pronounced as Saytzeff), in 1875, prepared a rule to help predict the result of elimination reactions which stated, "The favored product in dehydrohalogenation reactions is that alkene that has the majority of alkyl groups attached to the double-bonded carbon atoms."
Tosylate
Tosylates are important functional groups in organic chemistry, mainly because of two important properties which they possess:
Alkyl Halides
A functional group is a collection of several atoms or bonds with certain characteristic chemical properties and reactions associated with it. There is a presence of a halogen atom (F, Cl, Br, or I; it represents any halogen atom), as a functional group in alkyl halides. Therefore, it can be said that alkanes that contain a halogen compound are called alkyl halides.
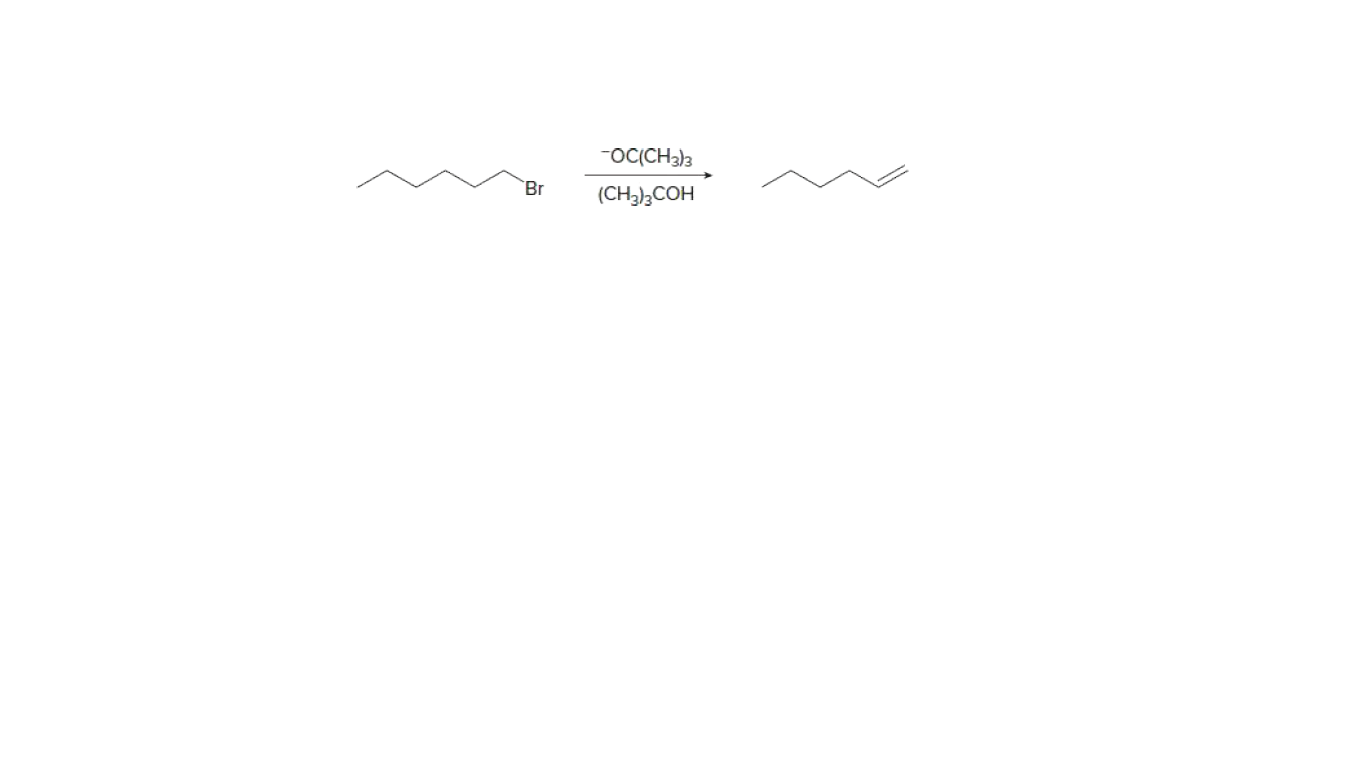
Consider the attached E2 reaction
What happens to the reaction rate with each of the following changes?
[1] The solvent is changed to DMF.
[2] The concentration of −OC(CH3)3 is decreased.
[3] The base is changed to −OH.
[4] The halide is changed to CH3CH2CH2CH2CH(Br)CH3.
[5] The leaving group is changed to I−.
Step by step
Solved in 7 steps with 1 images




