OH 1. aqueous H2SO4 CH3 CH3 2. H2SO4 OH H2O 130° a = Proton transfer d = El Elimination f= SN1 Nucleophilic substitution b = Lewis acid/base e = E2 Elimination g = SN2 Nucleophilic substitution c = Electrophilic addition The rections above involve synthesis or reactions of alcohols and ethers. Identify the mechanism by which they proceed from among the mechanisms listed. Use the letters a - g for your answers.
OH 1. aqueous H2SO4 CH3 CH3 2. H2SO4 OH H2O 130° a = Proton transfer d = El Elimination f= SN1 Nucleophilic substitution b = Lewis acid/base e = E2 Elimination g = SN2 Nucleophilic substitution c = Electrophilic addition The rections above involve synthesis or reactions of alcohols and ethers. Identify the mechanism by which they proceed from among the mechanisms listed. Use the letters a - g for your answers.
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
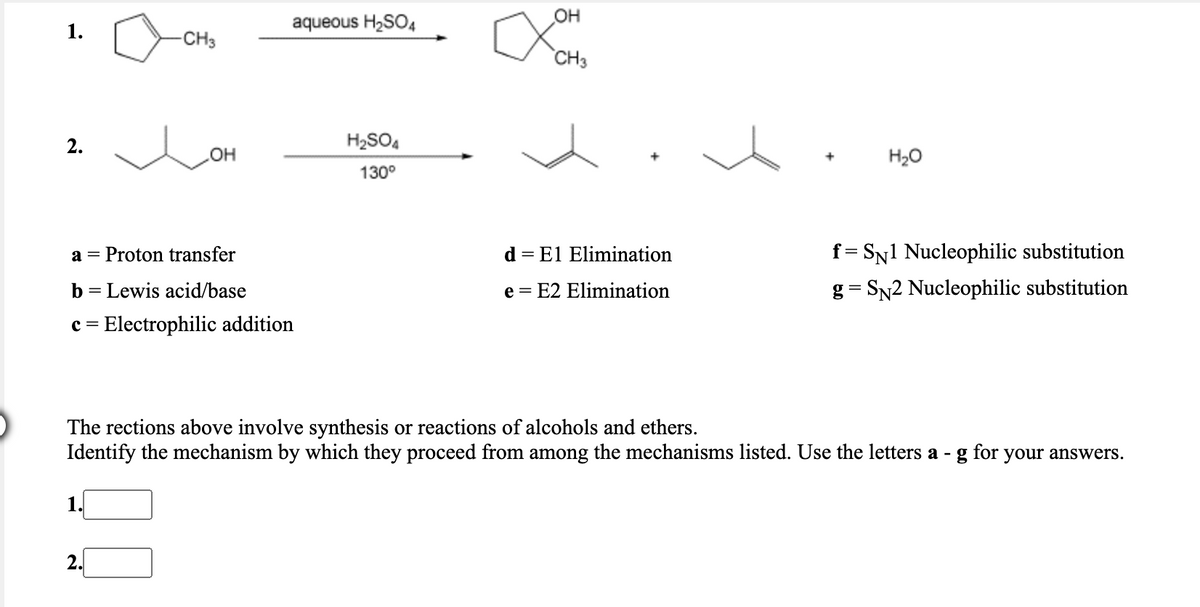
Transcribed Image Text:aqueous H2SO4
OH
1.
-CH3
CH3
2.
H2SO4
OH
H20
130°
Proton transfer
El Elimination
f= SN1 Nucleophilic substitution
a =
d =
b = Lewis acid/base
e = E2 Elimination
g= SN2 Nucleophilic substitution
c = Electrophilic addition
The rections above involve synthesis or reactions of alcohols and ethers.
Identify the mechanism by which they proceed from among the mechanisms listed. Use the letters a -
for
your answers.
1.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning