On an uncomfortable summer day, the air is at 87 °F and 80 % relative humidity. A laboratory air conditioner is to deliver 1.0 x 103 ft3/min of air at 55 °F in order to maintain the interior air at an average temperature of 75°F and a relative humidity of 40%. If the vent switch on the air conditioner is turned to the "open" position, outside air enters the unit as shown below. Assume A = 87 οF, B = 80 %, C = 55 οF. In the air conditioner, the air is cooled to a temperature low enough to condense the necessary amount of water and reheated to 55°F, at which point it has the same absolute humidity as the room air. Use the psychometric chart to estimate the rate (lbm/min) at which water is condensed, the temperature to which the air must be cooled to condense water at this rate, and the net tons of cooling required (Q⋅), where 1 ton of cooling = -12,000 Btu/h. [Note: The humid volume of the delivered air (at 55°F), which is difficult to read from the psychrometric chart, is 13.07 ft3/lbm dry air, and the heat capacity of liquid water is 1.0 Btu/(lbm °F).]
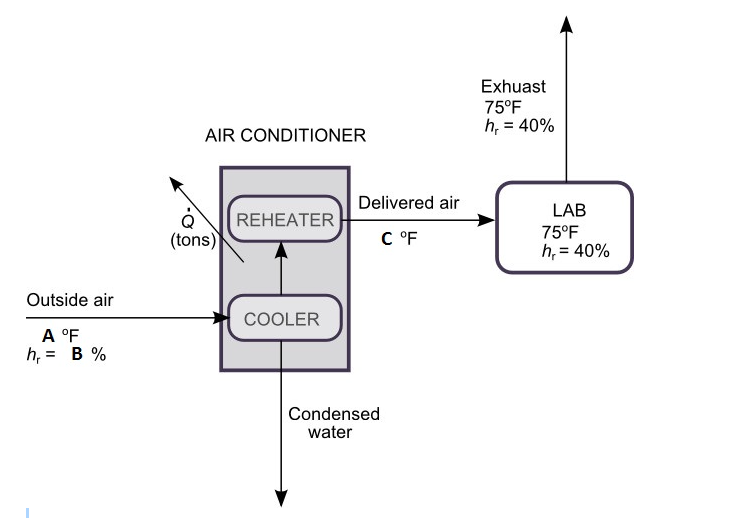
On an uncomfortable summer day, the air is at 87 °F and 80 % relative humidity. A laboratory air conditioner is to deliver 1.0 x 103 ft3/min of air at 55 °F in order to maintain the interior air at an average temperature of 75°F and a relative humidity of 40%.
If the vent switch on the air conditioner is turned to the "open" position, outside air enters the unit as shown below.
Assume A = 87 οF, B = 80 %, C = 55 οF.
In the air conditioner, the air is cooled to a temperature low enough to condense the necessary amount of water and reheated to 55°F, at which point it has the same absolute humidity as the room air.
Use the psychometric chart to estimate the rate (lbm/min) at which water is condensed, the temperature to which the air must be cooled to condense water at this rate, and the net tons of cooling required (Q⋅), where 1 ton of cooling = -12,000 Btu/h.
[Note: The humid volume of the delivered air (at 55°F), which is difficult to read from the psychrometric chart, is 13.07 ft3/lbm dry air, and the heat capacity of liquid water is 1.0 Btu/(lbm °F).]
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images









