on biac 1.5. If the temperature of a system of one mole of an ideal gas is changed from T1°C to T2 =2T1 °C is accompanied by a change in internal energy of 4000J, (a)Calculate the change in internal energy of the gas for doubling of the temperature at constant Volume. (b) Is there any heat supplied into or out of the system? (c) Does this quantity of heat correspond to the change in enthalpy of the gas.
on biac 1.5. If the temperature of a system of one mole of an ideal gas is changed from T1°C to T2 =2T1 °C is accompanied by a change in internal energy of 4000J, (a)Calculate the change in internal energy of the gas for doubling of the temperature at constant Volume. (b) Is there any heat supplied into or out of the system? (c) Does this quantity of heat correspond to the change in enthalpy of the gas.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.84E: Assume that 1.20 g of benzoicacid, C6H5COOH, is burned in a porcelain dish exposed to the air.If 31,...
Related questions
Question
1.5 please
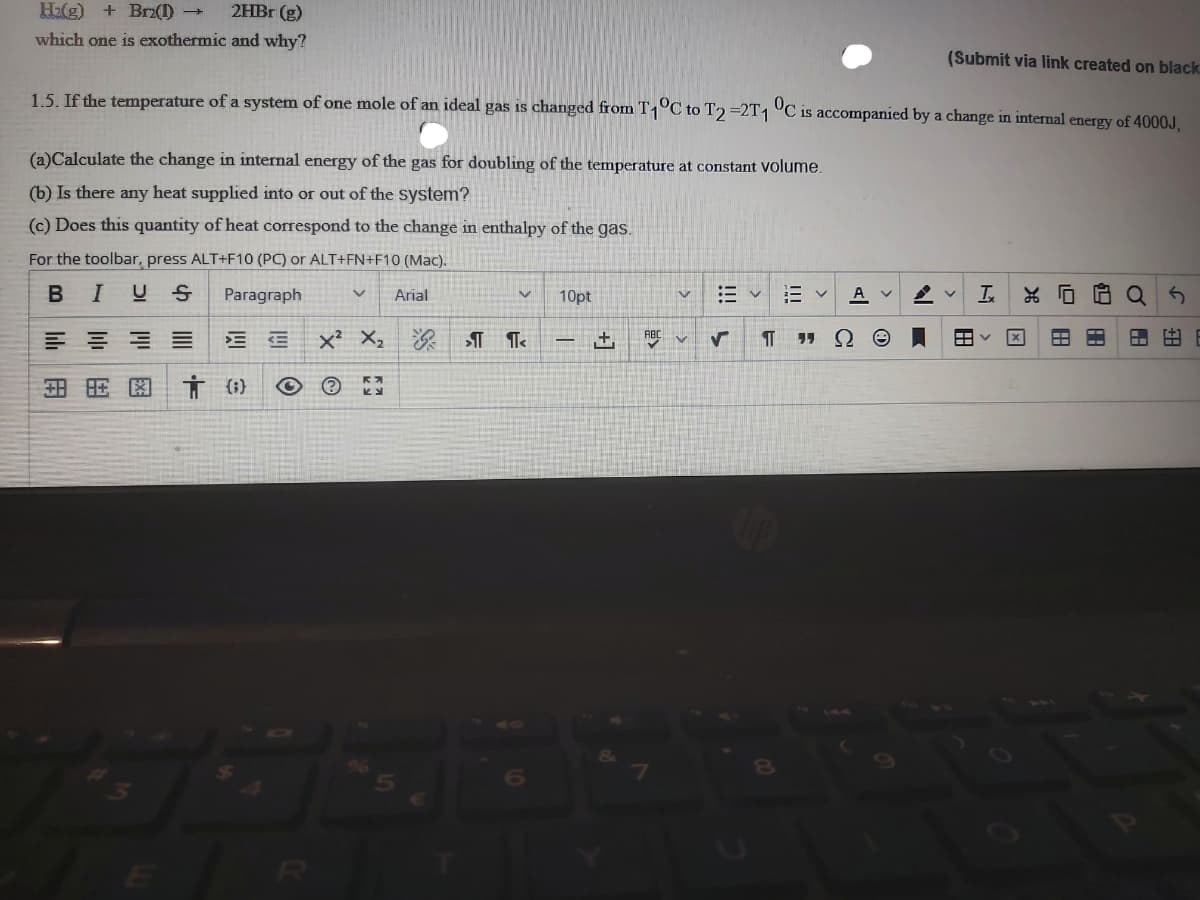
Transcribed Image Text:H2(g) + Brz(1) →
2HBr (g)
which one is exothermic and why?
(Submit via link created on black
1.5. If the temnperature of a system of one mole of an ideal gas is changed from T1°C to T2 =2T1 °C is accompanied by a change in internal energy of 4000J.
(a)Calculate the change in internal energy of the gas for doubling of the temperature at constant volume.
(b) Is there any heat supplied into or out of the system?
(c) Does this quantity of heat correspond to the change in enthalpy of the gas.
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
В I
U S
Paragraph
A v
Arial
10pt
x² X2
田
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning