Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section: Chapter Questions
Problem 4.15P: In each pair, select the stronger acid. (a) Pyruvic acid (pKa 2.49) or lactic acid (pKa 3.08) (b)...
Related questions
Question
Phthalic acid and isophthalic acid have protons on two carboxy groups that can be removed with base. (a) Explain why the pKa for loss of the first proton (pKa1) is lower for phthalic acid than isophthalic acid. (b) Explain why the pKa for loss of the second proton (pKa2) is higher for phthalic acid than isophthalic acid.
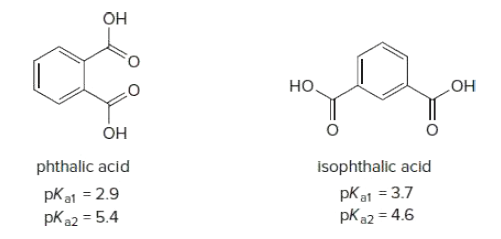
Transcribed Image Text:OH
но.
HO
phthalic acid
isophthalic acid
pKa1 = 2.9
pK22 = 5.4
pKa1 = 3.7
pKa2 = 4.6
Expert Solution
Step 1

Phthalic acid and isophthalic acid have protons on two carboxy groups that can be removed with base.
(a) Why the pKa for loss of the first proton (pKa1) is lower for phthalic acid than isophthalic acid has to be explained.
(b) Why the pKa for loss of the second proton (pKa2) is higher for phthalic acid than isophthalic acid has to be explained.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning