One of the steps to sweeten sour gas using the Claus process is reacting hydrogen sulfide gas with sulfur dioxide gas to produce water vapour and sulfur. 16 H,S(g) + 8 SO,(g) → 16 H,0(g) +3 Se(s) 8.43 kL of hydrogen sulfide at 175 kPa and 250 °C reacts with excess sulfur dioxide. Calculate the mass, in kg, of sulfur produced. Do not show your work in the space provided. Record only your final answer with the correct number of significant digits and the proper units. Answer:
One of the steps to sweeten sour gas using the Claus process is reacting hydrogen sulfide gas with sulfur dioxide gas to produce water vapour and sulfur. 16 H,S(g) + 8 SO,(g) → 16 H,0(g) +3 Se(s) 8.43 kL of hydrogen sulfide at 175 kPa and 250 °C reacts with excess sulfur dioxide. Calculate the mass, in kg, of sulfur produced. Do not show your work in the space provided. Record only your final answer with the correct number of significant digits and the proper units. Answer:
Chapter11: Organic Compounds: Alkanes
Section: Chapter Questions
Problem 11.74E
Related questions
Question

Transcribed Image Text:One of the steps to sweeten sour gas using the Claus process is reacting
hydrogen sulfide gas with sulfur dioxide gas to produce water vapour and
sulfur.
16 H,S(g) + 8 SO,(g) → 16 H,0(g) +3 Se(s)
8.43 kL of hydrogen sulfide at 175 kPa and 250 °C reacts with excess
sulfur dioxide. Calculate the mass, in kg, of sulfur produced.
Do not show your work in the space provided.
Record only your final answer with the correct number of significant digits
and the proper units.
Answer:
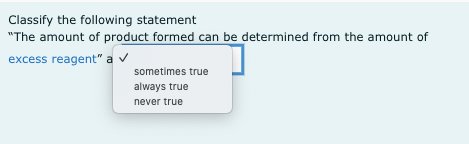
Transcribed Image Text:Classify the following statement
"The amount of product formed can be determined from the amount of
excess reagent" a v
sometimes true
always true
never true
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning