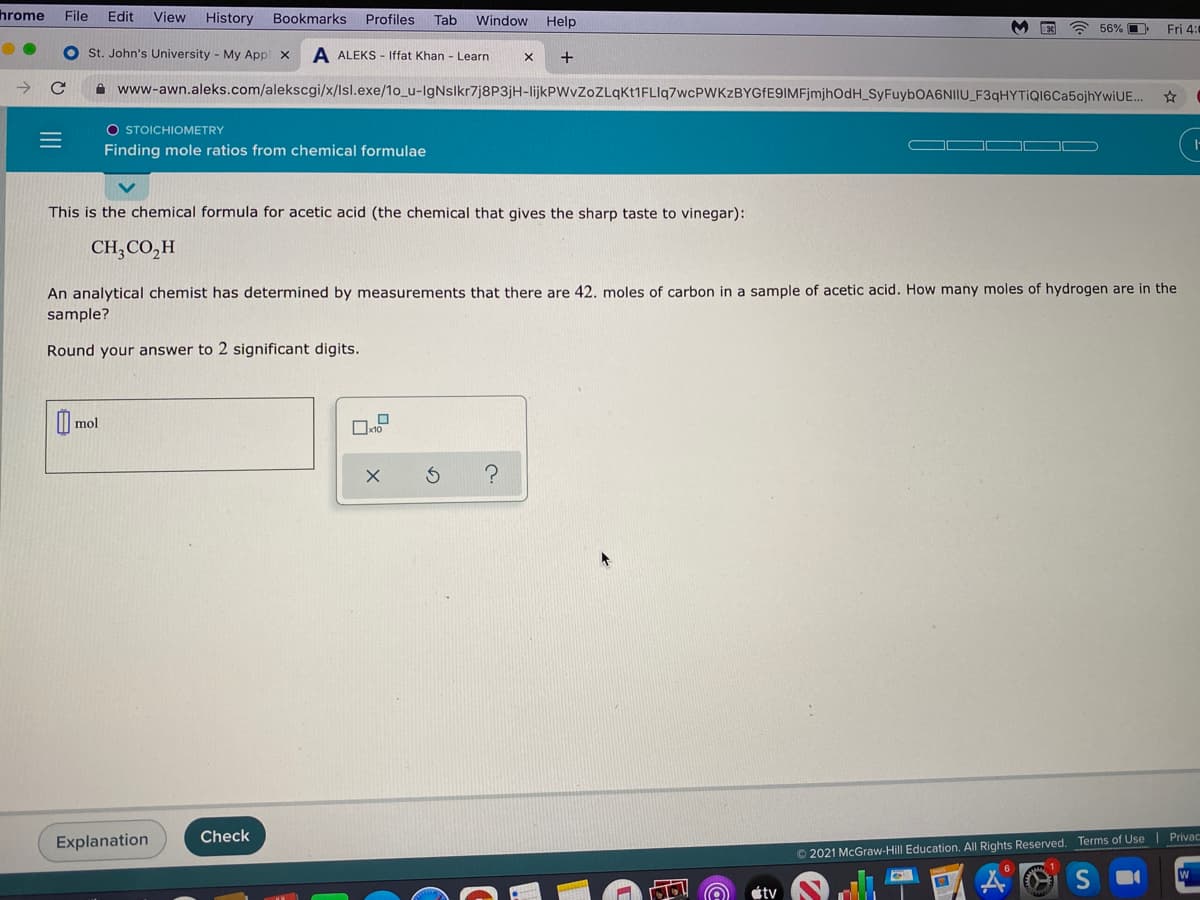
ONIIO_F3qHYTIQI6Ca5ojhYwiUE... O STOICHIOMETRY Finding mole ratios from chemical formulae This is the chemical formula for acetic acid (the chemical that gives the sharp taste to vinegar): CH;CO,H An analytical chemist has determined by measurements that there are 42. moles of carbon in a sample of acetic acid. How many moles of hydrogen are ir sample? Round your answer to 2 significant digits. I| mol
ONIIO_F3qHYTIQI6Ca5ojhYwiUE... O STOICHIOMETRY Finding mole ratios from chemical formulae This is the chemical formula for acetic acid (the chemical that gives the sharp taste to vinegar): CH;CO,H An analytical chemist has determined by measurements that there are 42. moles of carbon in a sample of acetic acid. How many moles of hydrogen are ir sample? Round your answer to 2 significant digits. I| mol
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 49A
Related questions
Question
Transcribed Image Text:hrome
File
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
56% O
Fri 4:
St. John's University - My Appl x
A ALEKS - Iffat Khan - Learn
+
A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFjmjhOdH_SyFuybOA6NIIU_F3qHYTiQI6Ca5ojhYwiUE..
O STOICHIOMETRY
Finding mole ratios from chemical formulae
This is the chemical formula for acetic acid (the chemical that gives the sharp taste to vinegar):
CH,CO,H
An analytical chemist has determined by measurements that there are 42. moles of carbon in a sample of acetic acid. How many moles of hydrogen are in the
sample?
Round your answer to 2 significant digits.
mol
Check
Explanation
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privac
étv
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
