9. A 0.1342-gram sample of an unknown compound composed of C, H, and O was burned in air, producing 0.240 g of CO2 and 0.0982 g of H2O. If the molar mass of the compound is 222.4 g/mole, determine its empirical and molecular formulas. c Cx Hy Ox + O2 The reston Scrap P
9. A 0.1342-gram sample of an unknown compound composed of C, H, and O was burned in air, producing 0.240 g of CO2 and 0.0982 g of H2O. If the molar mass of the compound is 222.4 g/mole, determine its empirical and molecular formulas. c Cx Hy Ox + O2 The reston Scrap P
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 81AP: For each of the following incomplete and unbalanced equations, indicate how many moles of the second...
Related questions
Question
My questions is about when we convert to moles in this problem. Why do we convert C and H to moles, then grams, and not say O and H and then find the grams of C from them. Or why can't we just find the moles of O from the given weight of H2O? Basically, why is O excluded in finding moles in the first part of the problem. I understand we need to subtract from the given weight 0.1342 grams but don't know why we can't just subtract g of O and grams of C why must it be C and H.
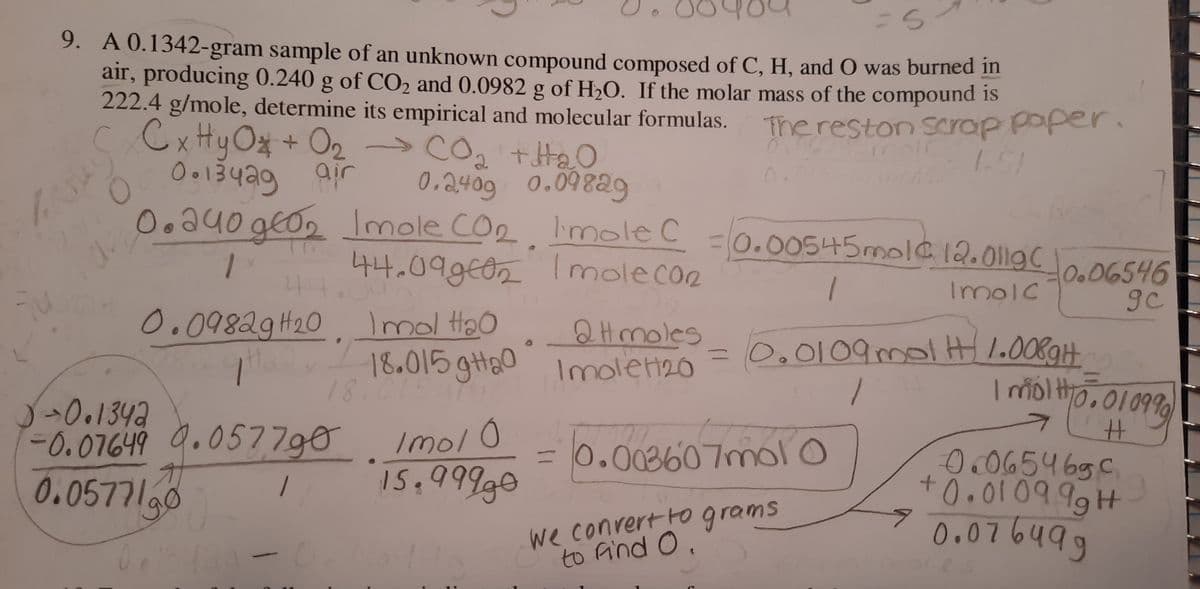
Transcribed Image Text:9. A 0.1342-gram sample of an unknown compound composed of C, H, and O was burned in
air, producing 0.240 g of CO2 and 0.0982 g of H2O. If the molar mass of the compound is
222.4 g/mole, determine its empirical and molecular formulas.
c Cx Hy O* + O2
O013429 air
0.a40 geo, lmale COp Imole C
The reston Scrap paper.
1.51
2
0.2409 0.098a9
0.
30.00545mole12.0119C
44.09g€02
Imolecoe
O.06546
gc
Imolc
0.09829H20
Imol Hao
Q Hmoles
Imolett20
=D0.0109mol .0089H
18.015 gH20
18.01
-0.07649 9.057790
0.0577l90
Imol0
I5.999g0
30.003607nmol o
O.O654 69C
+0.0109
we conrert Fograms
to Aind O.
0.076499
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
