Osmosis is the process of water molecules passing through a semipermeable membrane. If a semi-permipermeable membrane is separating a solution containin a 5.0 M NaCl aqueous solution and one containing a 0.75 M NaCl aqueous solutic what will happen? Assume that osmosis is the ONLY process which can occur. no water will flow through the semi-permeable membrane. Water will have a net flow into the 0.75 M solution, until equilibrium is reached Water will have a net flow into the 5.0 M solution, until equilibrium is reached.
Osmosis is the process of water molecules passing through a semipermeable membrane. If a semi-permipermeable membrane is separating a solution containin a 5.0 M NaCl aqueous solution and one containing a 0.75 M NaCl aqueous solutic what will happen? Assume that osmosis is the ONLY process which can occur. no water will flow through the semi-permeable membrane. Water will have a net flow into the 0.75 M solution, until equilibrium is reached Water will have a net flow into the 5.0 M solution, until equilibrium is reached.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.101QE
Related questions
Question
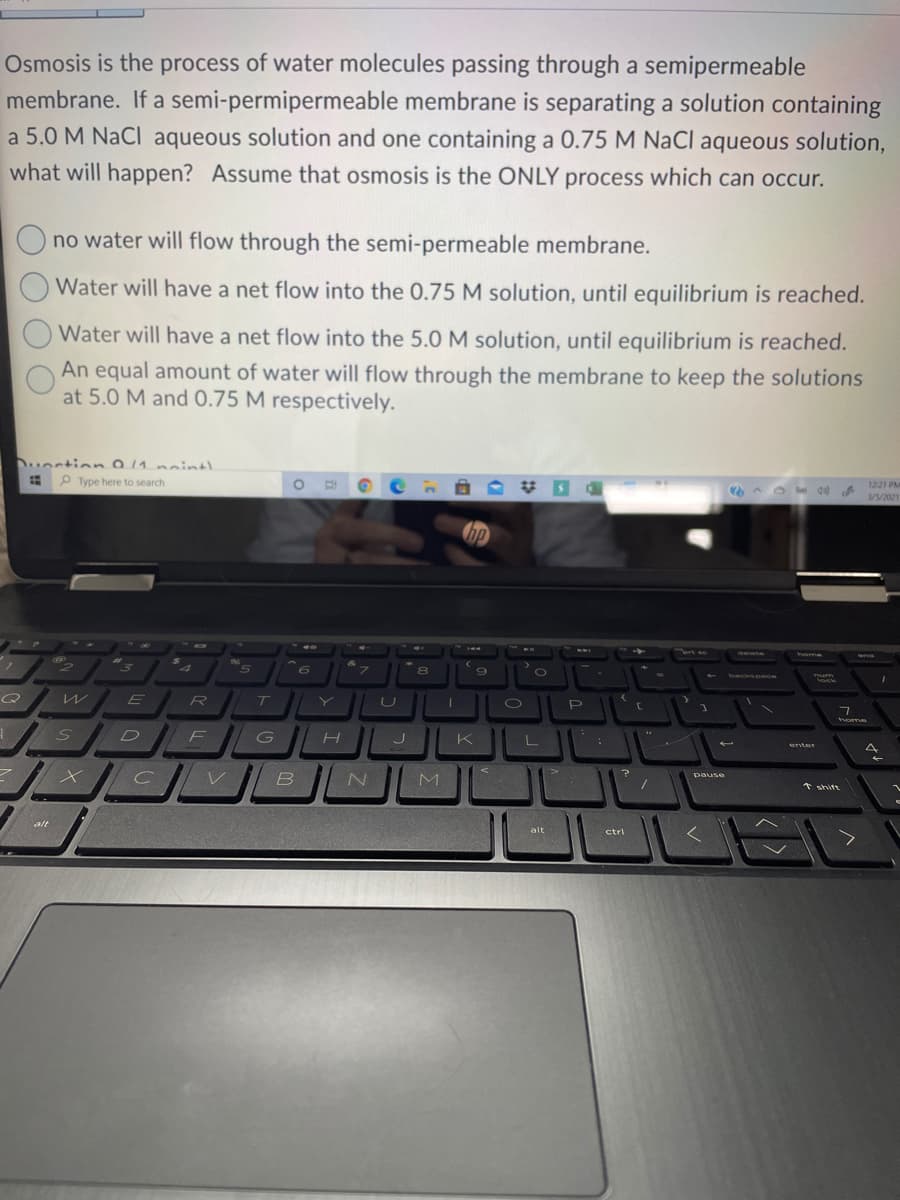
Transcribed Image Text:Osmosis is the process of water molecules passing through a semipermeable
membrane. If a semi-permipermeable membrane is separating a solution containing
a 5.0 M NaCl aqueous solution and one containing a 0.75 M NaCl aqueous solution,
what will happen? Assume that osmosis is the ONLY process which can occur.
no water will flow through the semi-permeable membrane.
Water will have a net flow into the 0.75 M solution, until equilibrium is reached.
Water will have a net flow into the 5.0 M solution, until equilibrium is reached.
An equal amount of water will flow through the membrane to keep the solutions
at 5.0 M and 0.75 M respectively.
Ducction011 noint)
P Type here to search
1221 PM
V/2021
144
ort sc
deiete
home
5.
backspace
lock
E
R
P
home
D
F
G
K
一
enter
pause
M
个hitt
alt
alt
ctri
O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning