P11C.15 The rotational constant for a diatomic molecule in the vibrational state with quantum number v typically fits the expression B, = B,- a(v+}), where B, is the rotational constant corresponding to the equilibrium bond length. For the interhalogen molecule IF it is found that B, = 0.279 71 cm and a = 0.187 m (note the change of units). Calculate B, and B, and use these values to calculate the wavenumbers of the transitions originating from J= 3 of the P and R branches. You will need the following additional information: v = 610.258 cm and x,ỹ = 3.141 cm. Estimate the dissociation energy of the IF molecule.
P11C.15 The rotational constant for a diatomic molecule in the vibrational state with quantum number v typically fits the expression B, = B,- a(v+}), where B, is the rotational constant corresponding to the equilibrium bond length. For the interhalogen molecule IF it is found that B, = 0.279 71 cm and a = 0.187 m (note the change of units). Calculate B, and B, and use these values to calculate the wavenumbers of the transitions originating from J= 3 of the P and R branches. You will need the following additional information: v = 610.258 cm and x,ỹ = 3.141 cm. Estimate the dissociation energy of the IF molecule.
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter11: Molecular Structure
Section: Chapter Questions
Problem 14P
Related questions
Question
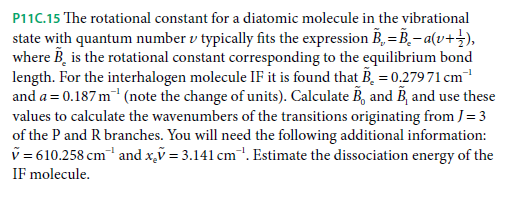
Transcribed Image Text:P11C.15 The rotational constant for a diatomic molecule in the vibrational
state with quantum number v typically fits the expression B, = B,- a(v+}),
where B, is the rotational constant corresponding to the equilibrium bond
length. For the interhalogen molecule IF it is found that B, = 0.279 71 cm
and a = 0.187 m (note the change of units). Calculate B, and B, and use these
values to calculate the wavenumbers of the transitions originating from J= 3
of the P and R branches. You will need the following additional information:
v = 610.258 cm and x,ỹ = 3.141 cm. Estimate the dissociation energy of the
IF molecule.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax