P1A.5 Deduce the relation between the pressure and mass density, p, of a perfect gas of molar mass M. Confirm graphically, using the following data on methoxymethane (dimethyl ether) at 25°C, that perfect behaviour is reached at low pressures and find the molar mass of the gas. p/kPa 12.223 25.20 36.97 60.37 85.23 101.3 p/(kgm) 0.225 0.456 0.664 1.062 1.468 1.734
P1A.5 Deduce the relation between the pressure and mass density, p, of a perfect gas of molar mass M. Confirm graphically, using the following data on methoxymethane (dimethyl ether) at 25°C, that perfect behaviour is reached at low pressures and find the molar mass of the gas. p/kPa 12.223 25.20 36.97 60.37 85.23 101.3 p/(kgm) 0.225 0.456 0.664 1.062 1.468 1.734
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.51E: Numerically evaluate for one mole of methane acting as a van der Waals gas at a T = 298 K and V =...
Related questions
Question
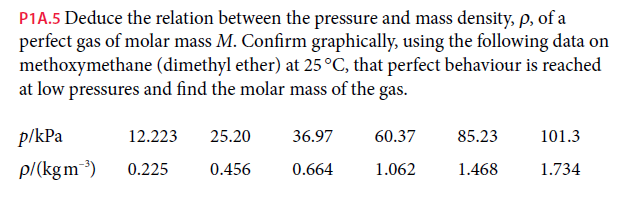
Transcribed Image Text:P1A.5 Deduce the relation between the pressure and mass density, p, of a
perfect gas of molar mass M. Confirm graphically, using the following data on
methoxymethane (dimethyl ether) at 25°C, that perfect behaviour is reached
at low pressures and find the molar mass of the gas.
p/kPa
12.223
25.20
36.97
60.37
85.23
101.3
p/(kgm)
0.225
0.456
0.664
1.062
1.468
1.734
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning