P3D.2 In biological cells, the energy released by the oxidation of foods is stored in adenosine triphosphate (ATP or ATP“).The essence of ATP's action is its ability to lose its terminal phosphate group by hydrolysis and to form adenosine diphosphate (ADP or ADP): ATP* (aq) + H,O() → ADP* (aq) + HPO (aq) + H,O*(aq) At pH = 7.0 and 37°C (310K, blood temperature) the enthalpy and Gibbs energy of hydrolysis are A,H =-20kJ mol and A,G=-31 kJ mol", respectively. Under these conditions, the hydrolysis of 1 mol ATP“(aq) results in the extraction of up to 31kJ of energy that can be used to do non- expansion work, such as the synthesis of proteins from amino acids, muscular contraction, and the activation of neuronal circuits in our brains. (a) Calculate and account for the sign of the entropy of hydrolysis of ATP at pH = 7.0 and 310K. (b) Suppose that the radius of a typical biological cell is 10µm and that inside it 1x 10ʻ ATP molecules are hydrolysed each second. What is the power density of the cell in watts per cubic metre (1 W = 1 J s')? A computer battery delivers about 15 W and has a volume of 100 cm'. Which has the greater power density, the cell or the battery? (c) The formation of glutamine from glutamate and ammonium ions requires 14.2kJ mol of energy input. It is driven by the hydrolysis of ATP to ADP mediated by the enzyme glutamine synthetase. How many moles of ATP must be hydrolysed to form 1 mol glutamine?
P3D.2 In biological cells, the energy released by the oxidation of foods is stored in adenosine triphosphate (ATP or ATP“).The essence of ATP's action is its ability to lose its terminal phosphate group by hydrolysis and to form adenosine diphosphate (ADP or ADP): ATP* (aq) + H,O() → ADP* (aq) + HPO (aq) + H,O*(aq) At pH = 7.0 and 37°C (310K, blood temperature) the enthalpy and Gibbs energy of hydrolysis are A,H =-20kJ mol and A,G=-31 kJ mol", respectively. Under these conditions, the hydrolysis of 1 mol ATP“(aq) results in the extraction of up to 31kJ of energy that can be used to do non- expansion work, such as the synthesis of proteins from amino acids, muscular contraction, and the activation of neuronal circuits in our brains. (a) Calculate and account for the sign of the entropy of hydrolysis of ATP at pH = 7.0 and 310K. (b) Suppose that the radius of a typical biological cell is 10µm and that inside it 1x 10ʻ ATP molecules are hydrolysed each second. What is the power density of the cell in watts per cubic metre (1 W = 1 J s')? A computer battery delivers about 15 W and has a volume of 100 cm'. Which has the greater power density, the cell or the battery? (c) The formation of glutamine from glutamate and ammonium ions requires 14.2kJ mol of energy input. It is driven by the hydrolysis of ATP to ADP mediated by the enzyme glutamine synthetase. How many moles of ATP must be hydrolysed to form 1 mol glutamine?
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 15P
Related questions
Question
100%
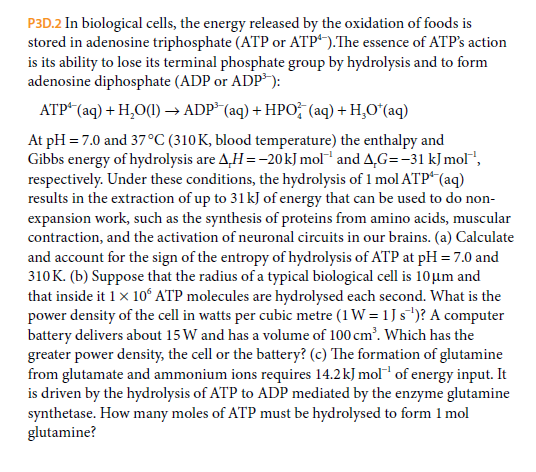
Transcribed Image Text:P3D.2 In biological cells, the energy released by the oxidation of foods is
stored in adenosine triphosphate (ATP or ATP“).The essence of ATP's action
is its ability to lose its terminal phosphate group by hydrolysis and to form
adenosine diphosphate (ADP or ADP):
ATP* (aq) + H,O() → ADP* (aq) + HPO (aq) + H,O*(aq)
At pH = 7.0 and 37°C (310K, blood temperature) the enthalpy and
Gibbs energy of hydrolysis are A,H =-20kJ mol and A,G=-31 kJ mol",
respectively. Under these conditions, the hydrolysis of 1 mol ATP“(aq)
results in the extraction of up to 31kJ of energy that can be used to do non-
expansion work, such as the synthesis of proteins from amino acids, muscular
contraction, and the activation of neuronal circuits in our brains. (a) Calculate
and account for the sign of the entropy of hydrolysis of ATP at pH = 7.0 and
310K. (b) Suppose that the radius of a typical biological cell is 10µm and
that inside it 1x 10ʻ ATP molecules are hydrolysed each second. What is the
power density of the cell in watts per cubic metre (1 W = 1 J s')? A computer
battery delivers about 15 W and has a volume of 100 cm'. Which has the
greater power density, the cell or the battery? (c) The formation of glutamine
from glutamate and ammonium ions requires 14.2kJ mol of energy input. It
is driven by the hydrolysis of ATP to ADP mediated by the enzyme glutamine
synthetase. How many moles of ATP must be hydrolysed to form 1 mol
glutamine?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax