P5B.7 The molar mass of a protein was determined by dissolving it in water, and measuring the height, h, of the resulting solution drawn up a capillary tube at 20°C. The following data were obtained. c/(mg cm) 3.221 4.618 5.112 6.722 h/cm 5.746 8.238 9.119 11.990 The osmotic pressure may be calculated from the height of the column as Il=hpg, taking the mass density of the solution as p=1.000 gcm³and the acceleration of free fall as g=9.81 ms. Determine the molar mass of the protein.
P5B.7 The molar mass of a protein was determined by dissolving it in water, and measuring the height, h, of the resulting solution drawn up a capillary tube at 20°C. The following data were obtained. c/(mg cm) 3.221 4.618 5.112 6.722 h/cm 5.746 8.238 9.119 11.990 The osmotic pressure may be calculated from the height of the column as Il=hpg, taking the mass density of the solution as p=1.000 gcm³and the acceleration of free fall as g=9.81 ms. Determine the molar mass of the protein.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 119QRT
Related questions
Question
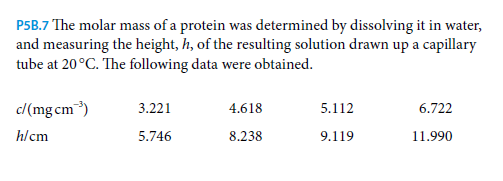
Transcribed Image Text:P5B.7 The molar mass of a protein was determined by dissolving it in water,
and measuring the height, h, of the resulting solution drawn up a capillary
tube at 20°C. The following data were obtained.
c/(mg cm)
3.221
4.618
5.112
6.722
h/cm
5.746
8.238
9.119
11.990

Transcribed Image Text:The osmotic pressure may be calculated from the height of the column as
Il=hpg, taking the mass density of the solution as p=1.000 gcm³and the
acceleration of free fall as g=9.81 ms. Determine the molar mass of the
protein.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning