Part 1- Calculations: To calculate the exact concentration of your ~0.1 M NAOH(aq) solution, the following logic stream can be used. The stockroom will provide you with a stock solution of KHP(aq), whose concentration is known and labeled on the bottle. Be sure to record this concentration, with proper units, in the data section of your notebook! use 25.00 mL of KHP solution concentration of KHP mass of KHP (grams) moles of KHP grams KHP(s) liters of solution 1:1 mole ratio of KHP:NaOH from balanced chemical equation molarity of NaOH(aq) volume of NaOH(aq) (mL) moles NaOH moles of NaOH liters of solution added to neutralize KHP
Part 1- Calculations: To calculate the exact concentration of your ~0.1 M NAOH(aq) solution, the following logic stream can be used. The stockroom will provide you with a stock solution of KHP(aq), whose concentration is known and labeled on the bottle. Be sure to record this concentration, with proper units, in the data section of your notebook! use 25.00 mL of KHP solution concentration of KHP mass of KHP (grams) moles of KHP grams KHP(s) liters of solution 1:1 mole ratio of KHP:NaOH from balanced chemical equation molarity of NaOH(aq) volume of NaOH(aq) (mL) moles NaOH moles of NaOH liters of solution added to neutralize KHP
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Hi I am having trouble finishing and finding the rest of the data for my Titration lab. I've already imputed the given data and just need to find the rest of the excel boxes using those data, thank you.
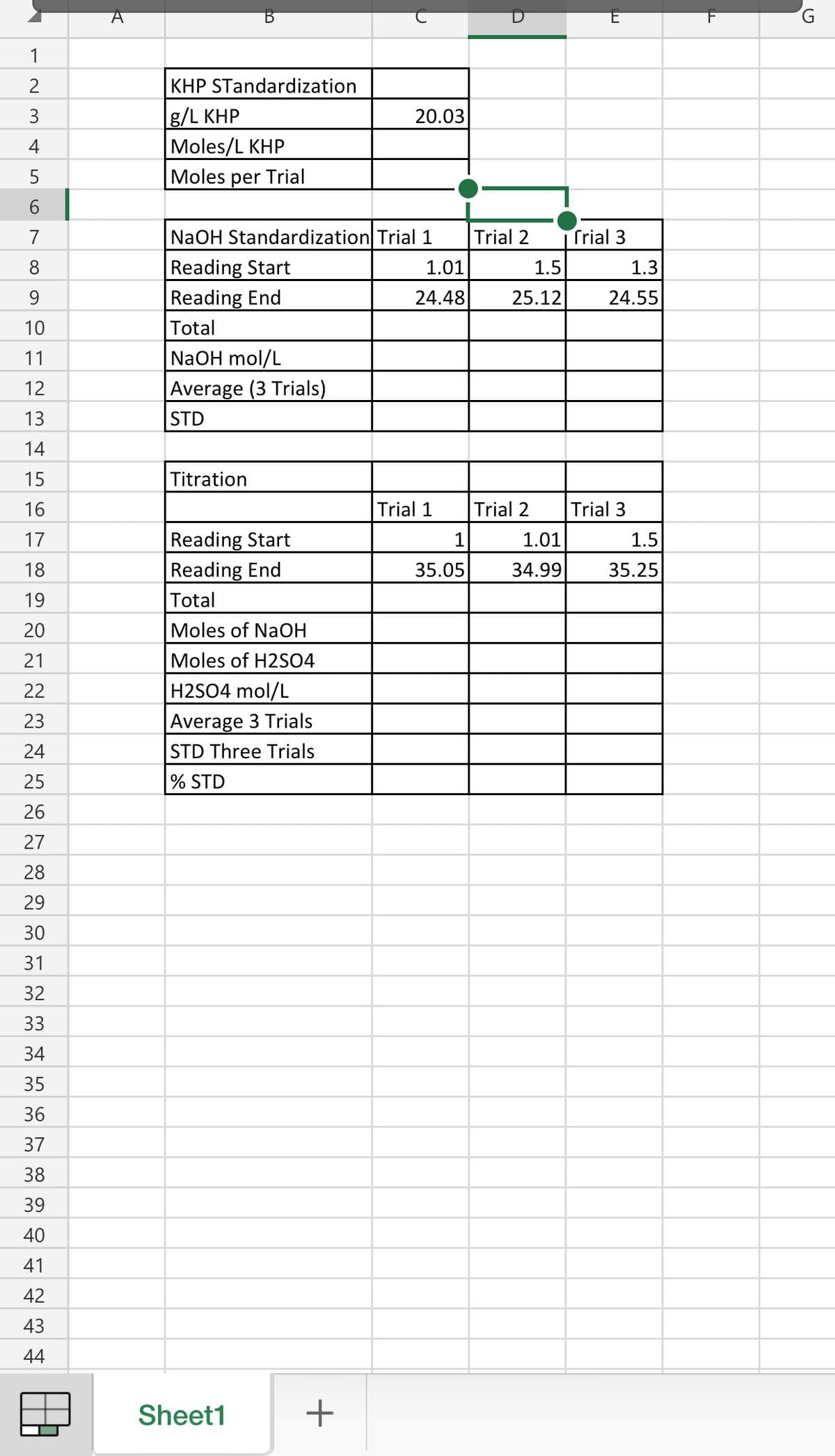
Transcribed Image Text:1
2
KHP STandardization
g/L KHP
Moles/L KHP
3
20.03
4
5
Moles per Trial
6
7
NaOH Standardization Trial 1
Trial 2
Trial 3
Reading Start
Reading End
8
1.01
1.5
1.3
9
24.48
25.12
24.55
10
Total
11
NaOH mol/L
12
Average (3 Trials)
13
STD
14
15
Titration
16
Trial 1
Trial 2
Trial 3
Reading Start
Reading End
17
1
1.01
1.5
18
35.05
34.99
35.25
19
Total
20
Moles of NaOH
21
Moles of H2SO4
22
H2SO4 mol/L
Average 3 Trials
STD Three Trials
% STD
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
Sheet1
+
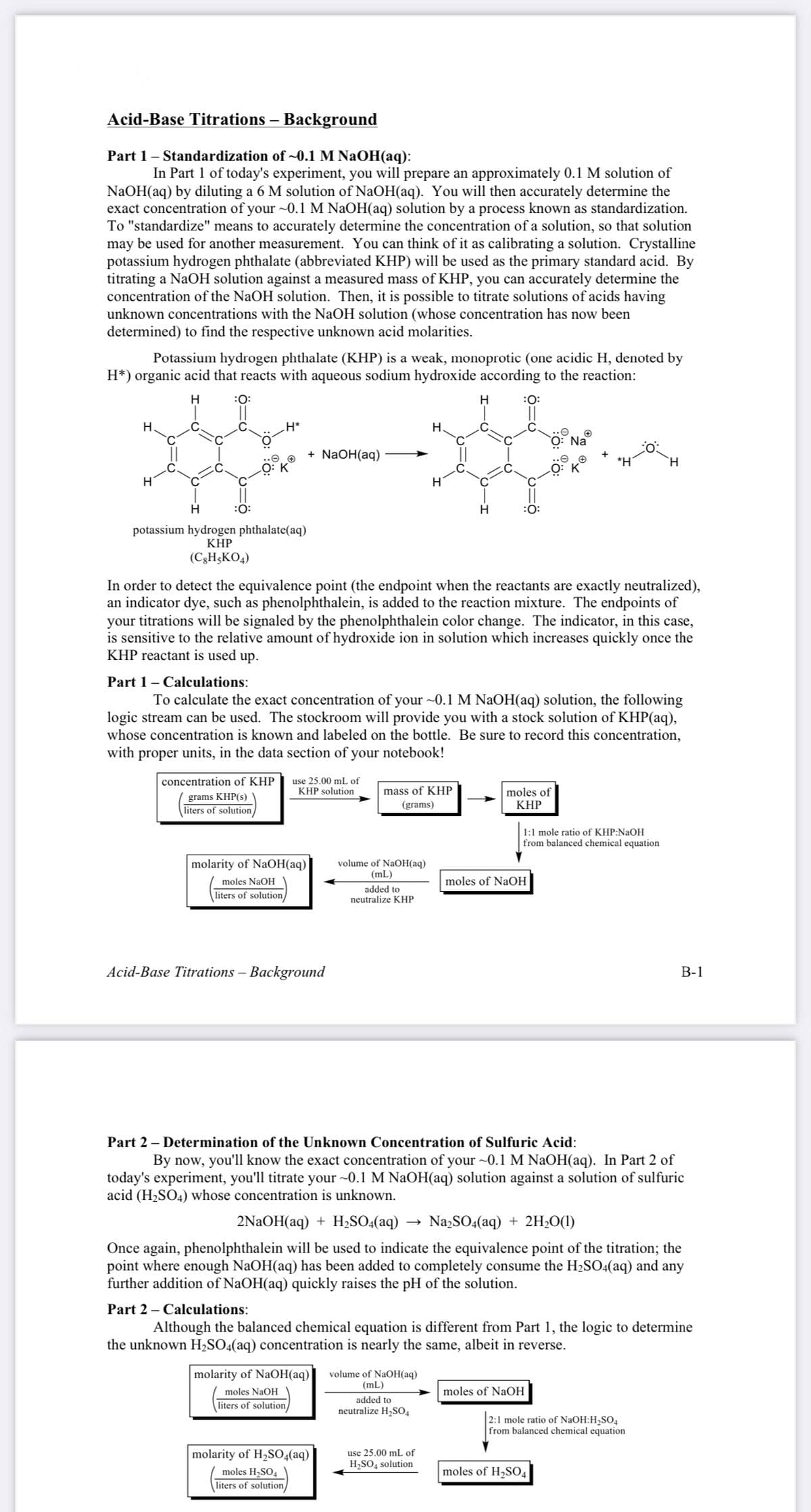
Transcribed Image Text:Acid-Base Titrations – Background
Part 1– Standardization of ~0.1 M NaOH(aq):
In Part 1 of today's experiment, you will prepare an approximately 0.1 M solution of
NaOH(aq) by diluting a 6 M solution of NaOH(aq). You will then accurately determine the
exact concentration of your ~0.1 M NAOH(aq) solution by a process known as standardization.
To "standardize" means to accurately determine the concentration of a solution, so that solution
may be used for another measurement. You can think of it as calibrating a solution. Crystalline
potassium hydrogen phthalate (abbreviated KHP) will be used as the primary standard acid. By
titrating a NaOH solution against a measured mass of KHP, you can accurately determine the
concentration of the NaOH solution. Then, it is possible to titrate solutions of acids having
unknown concentrations with the NaOH solution (whose concentration has now been
determined) to find the respective unknown acid molarities.
Potassium hydrogen phthalate (KHP) is a weak, monoprotic (one acidic H, denoted by
H*) organic acid that reacts with aqueous sodium hydroxide according to the reaction:
H
:0:
H
:0:
H
H*
Н.
Na
+ NaOH(aq)
*H'
`H
H
:0:
potassium hydrogen phthalate(aq)
KHP
(C3H;KO4)
In order to detect the equivalence point (the endpoint when the reactants are exactly neutralized),
an indicator dye, such as phenolphthalein, is added to the reaction mixture. The endpoints of
your titrations will be signaled by the phenolphthalein color change. The indicator, in this case,
is sensitive to the relative amount of hydroxide ion in solution which increases quickly once the
KHP reactant is used up.
Part 1- Calculations:
To calculate the exact concentration of your ~0.1 M NAOH(aq) solution, the following
logic stream can be used. The stockroom will provide you with a stock solution of KHP(aq),
whose concentration is known and labeled on the bottle. Be sure to record this concentration,
with proper units, in the data section of your notebook!
use 25.00 mL of
KHP solution
concentration of KHP
mass of KHP
grams KHP(s)
liters of solution/
moles of
KHP
(grams)
1:1 mole ratio of KHP:NaOH
from balanced chemical equation
molarity of NaOH(aq)
volume of NaOH(aq)
(mL)
moles NaOH
moles of NaOH
liters of solution/
added to
neutralize KHP
Acid-Base Titrations – Background
В-1
Part 2 – Determination of the Unknown Concentration of Sulfuric Acid:
By now, you'll know the exact concentration of your ~0.1 M NaOH(aq). In Part 2 of
today's experiment, you'll titrate your ~0.1 M NaOH(aq) solution against a solution of sulfuric
acid (H2SO4) whose concentration is unknown.
2NAOH(aq) + H;S0:(aq)
Na2SO4(aq) + 2H;O(1)
Once again, phenolphthalein will be used to indicate the equivalence point of the titration; the
point where enough NaOH(aq) has been added to completely consume the H2SO4(aq) and any
further addition of NaOH(aq) quickly raises the pH of the solution.
Part 2 – Calculations:
Although the balanced chemical equation is different from Part 1, the logic to determine
the unknown H,SO4(aq) concentration is nearly the same, albeit in reverse.
molarity of NaOH(aq)
volume of NaOH(aq)
(mL)
moles NaOH
moles of NaOH
liters of solution/
added to
neutralize H,SO4
2:1 mole ratio of NaOH:H2SO4
from balanced chemical equation
molarity of H,SO4(aq)
moles H2SO4
liters of solution/
use 25.00 mL of
H,SO, solution
moles of H2SO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning